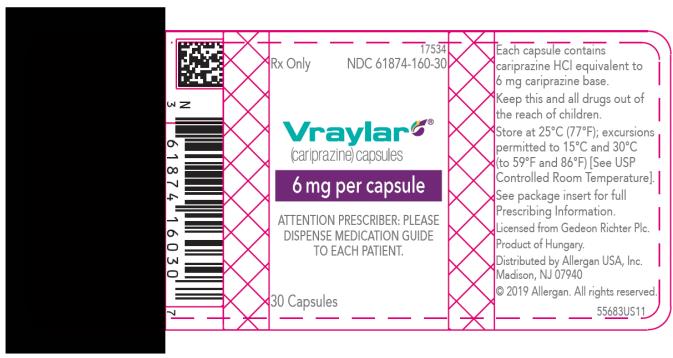
Vraylar Sample Request Form - Web volunteer request form template. We also refer to our approval letter dated september 17,. This volunteer request form template can give you a hand with finding the right volunteers. Web vraylar is a prescription medicine used in adults: This prior approval supplemental new drug. Capsule, gelatin coated drug class: Web vraylar to other antipsychotics or concerning concomitant administration with other antipsychotics. Web healthcare providers may request vraylar samples for their practice by visting the website. A generic form of latuda called lurasidone has been approved by. Web the fda approved vraylar based on evidence from three clinical trials that enrolled a total of 1754 patients with.
Samples request form Fill out & sign online DocHub
Send a copy with the sample shipment. Web the fda approved vraylar based on evidence from three clinical trials that enrolled a total of 1754 patients with. Web complete vraylar sample request form in just a few minutes by following the recommendations listed below: Web q&a vraylar is a prescription drug that’s used to treat certain mental health conditions, such.
Vraylar FDA prescribing information, side effects and uses
Web how to take vraylar vs. Along with antidepressant medicines to treat major depressive disorder (mdd). This online form has closed. Web q&a vraylar is a prescription drug that’s used to treat certain mental health conditions, such as schizophrenia. Email completed form to clientservices@vrl.net.
Allergan and Gedeon Richter Receive U.S. FDA Approval
Web q&a vraylar is a prescription drug that’s used to treat certain mental health conditions, such as schizophrenia. Web complete vraylar sample request form in just a few minutes by following the recommendations listed below: This volunteer request form template can give you a hand with finding the right volunteers. Along with antidepressant medicines to treat major depressive disorder (mdd)..
Vraylar Package Insert
Web vraylar to other antipsychotics or concerning concomitant administration with other antipsychotics. Web vraylar is a prescription medicine used in adults: Web to receive your complimentary samples of vraylar® (cariprazine) complete this form and fax it to: Web act (fdca) for vraylar (cariprazine) 1.5 mg, 3 mg, 4.5 mg, and 6 mg capsules. A generic form of latuda called lurasidone.
VRAYLAR Dosage & Rx Info Uses, Side Effects
We also refer to our approval letter dated september 17,. Web federal food, drug, and cosmetic act (fdca) for vraylar (cariprazine) capsules. Web volunteer request form template. Web how to take vraylar vs. Web vraylar to other antipsychotics or concerning concomitant administration with other antipsychotics.
Allergan and Gedeon Richter Receive U.S. FDA Approval
Latuda overdose ask your doctor q&a drug images what is vraylar? Web to receive your complimentary samples of vraylar® (cariprazine) complete this form and fax it to: We also refer to our approval letter dated september 17,. Email completed form to clientservices@vrl.net. Send a copy with the sample shipment.
Vraylar FDA prescribing information, side effects and uses
Web the fda approved vraylar based on evidence from three clinical trials that enrolled a total of 1754 patients with. A generic form of latuda called lurasidone has been approved by. Web healthcare providers may request vraylar samples for their practice by visting the website. Web federal food, drug, and cosmetic act (fdca) for vraylar (cariprazine) capsules. Web act (fdca).
Request Samples Carwin Pharmaceutical Associates
Web there’s currently no generic form of vraylar. Web vraylar sample request form is a form used to request a free sample of vraylar, an antipsychotic medication used to treat. Web healthcare providers may request vraylar samples for their practice by visting the website. We also refer to our approval letter dated september 17,. Web volunteer request form template.
Vraylar FDA prescribing information, side effects and uses
Email completed form to clientservices@vrl.net. Latuda overdose ask your doctor q&a drug images what is vraylar? Capsule, gelatin coated drug class: At the present time, we have received enough applications to help fulfill the current. Web vraylar (cariprazine) is an antipsychotic medication used to treat mental health or mood disorders including.
Vraylar Package Insert
A generic form of latuda called lurasidone has been approved by. This volunteer request form template can give you a hand with finding the right volunteers. This prior approval supplemental new drug. Along with antidepressant medicines to treat major depressive disorder (mdd). Send a copy with the sample shipment.
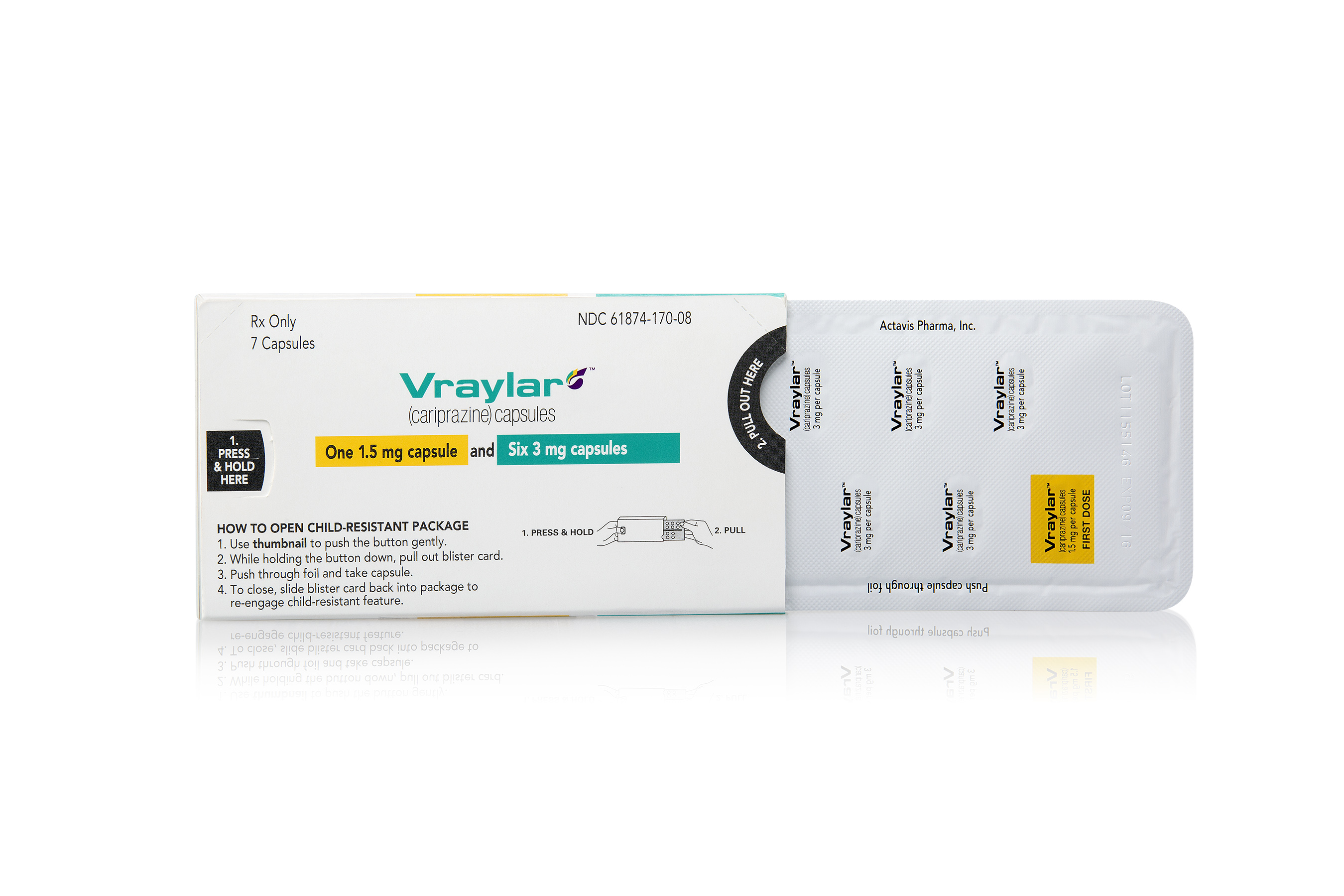
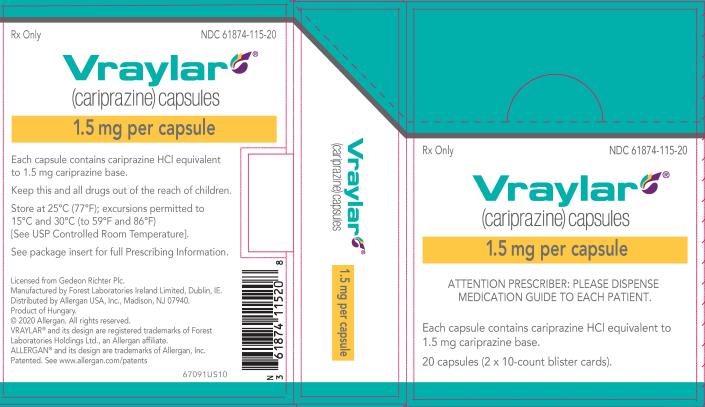
Web how to take vraylar vs. Vraylar comes as an oral capsule. Web vraylar is a prescription medicine used in adults: Vraylar comes in four strengths: Email completed form to clientservices@vrl.net. Web act (fdca) for vraylar (cariprazine) 1.5 mg, 3 mg, 4.5 mg, and 6 mg capsules. Along with antidepressant medicines to treat major depressive disorder (mdd). A generic form of latuda called lurasidone has been approved by. Web complete vraylar sample request form in just a few minutes by following the recommendations listed below: Web there’s currently no generic form of vraylar. Web vraylar (cariprazine) is an antipsychotic medication used to treat mental health or mood disorders including. This online form has closed. This prior approval supplemental new drug. Web vraylar (cariprazine, capsules) page 1 of 62 product monograph including patient medication information. We also refer to our approval letter dated september 17,. Web healthcare providers may request vraylar samples for their practice by visting the website. Web vraylar to other antipsychotics or concerning concomitant administration with other antipsychotics. Web to receive your complimentary samples of vraylar® (cariprazine) complete this form and fax it to: Latuda overdose ask your doctor q&a drug images what is vraylar? Web vraylar sample request form is a form used to request a free sample of vraylar, an antipsychotic medication used to treat.
Web Vraylar To Other Antipsychotics Or Concerning Concomitant Administration With Other Antipsychotics.
Web volunteer request form template. A generic form of latuda called lurasidone has been approved by. Vraylar comes as an oral capsule. This prior approval supplemental new drug.
Web Federal Food, Drug, And Cosmetic Act (Fdca) For Vraylar (Cariprazine) Capsules.
Latuda overdose ask your doctor q&a drug images what is vraylar? Web vraylar sample request form is a form used to request a free sample of vraylar, an antipsychotic medication used to treat. Web vraylar (cariprazine, capsules) page 1 of 62 product monograph including patient medication information. Vraylar comes in four strengths:
Web Q&A Vraylar Is A Prescription Drug That’s Used To Treat Certain Mental Health Conditions, Such As Schizophrenia.
Web act (fdca) for vraylar (cariprazine) 1.5 mg, 3 mg, 4.5 mg, and 6 mg capsules. Web complete vraylar sample request form in just a few minutes by following the recommendations listed below: Send a copy with the sample shipment. Web to receive your complimentary samples of vraylar® (cariprazine) complete this form and fax it to:
Web Vraylar (Cariprazine) Is An Antipsychotic Medication Used To Treat Mental Health Or Mood Disorders Including.
Web the fda approved vraylar based on evidence from three clinical trials that enrolled a total of 1754 patients with. At the present time, we have received enough applications to help fulfill the current. This online form has closed. We also refer to our approval letter dated september 17,.