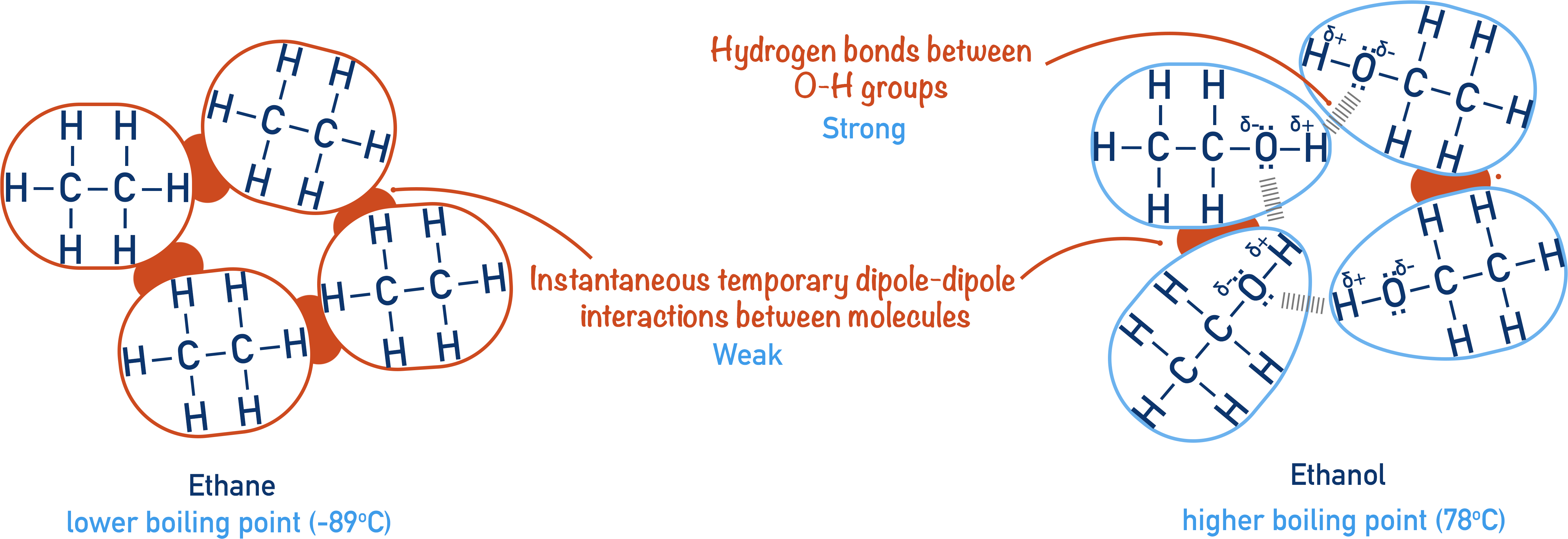
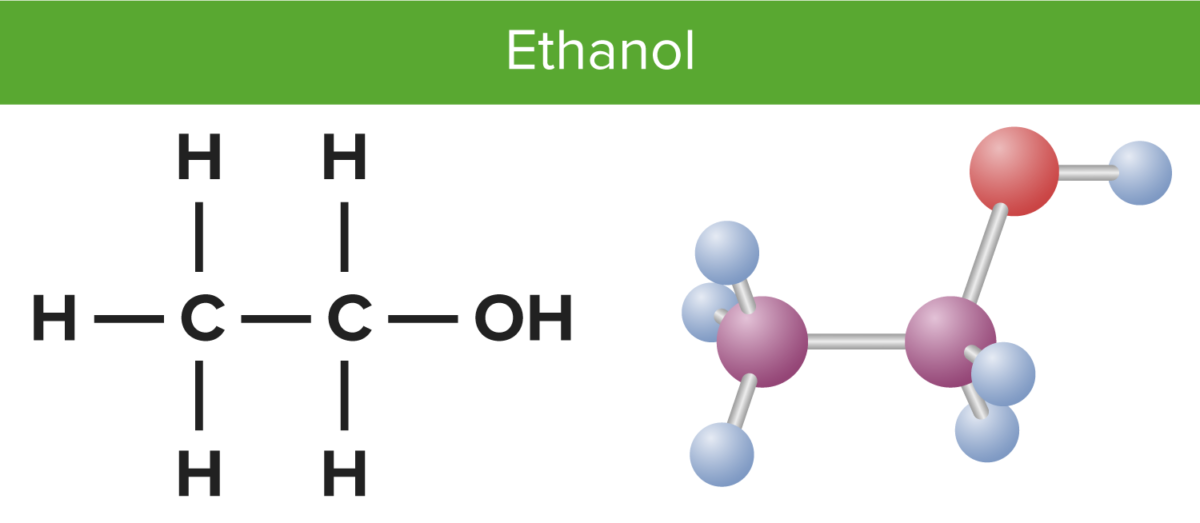
Will Ethane Form A Hydrogen Bond With Water - Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because. Web water is an even better example of a hydrogen bonding compound; Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web answer (1 of 3): Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that. Web hydrogen bonding plays a crucial role in many biological processes and can account for many natural phenomena. Web 1 answer sorted by:
Alcohols (ALevel) ChemistryStudent
Web this means the molecules will be soluble in a polar solvent such as water. Methane is a gas, and so its. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because. Web answer (1 of.
Pin on Hydrogen
What is the intramolecular bond that holds the hydrogen and carbon atoms within an. Methane is a gas, and so its. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web answer (1 of 3): Web 1 answer sorted by:
Hydrogen Bonds — Overview & Examples Expii
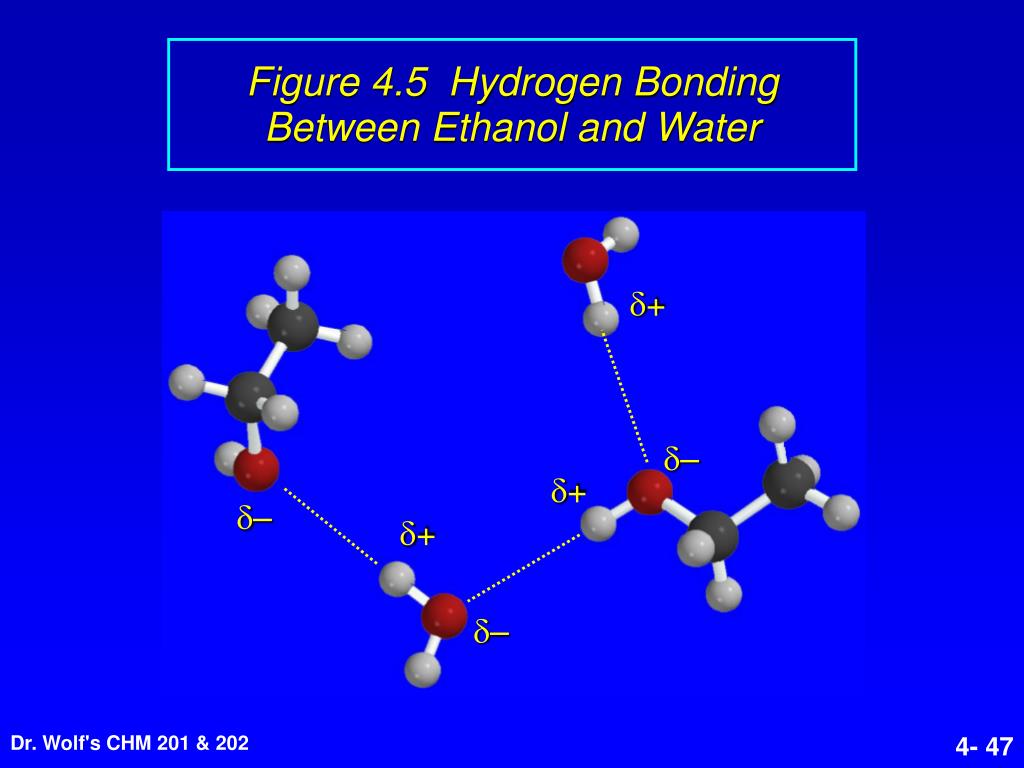
Web intermolecular force of two polar water molecules forming a hydrogen bond. The methane, ch 4, itself is not the problem. In fact, water molecules are so tightly bound to each. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. What is the intramolecular bond that holds the hydrogen and carbon atoms.
PPT Chapter 4 Alcohols and Alkyl Halides PowerPoint Presentation
Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. All of the bonds between multiple water. Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen.
PPT Bonding theory PowerPoint Presentation, free download ID2201847
Web intermolecular force of two polar water molecules forming a hydrogen bond. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because. Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain. In fact, water molecules are so.
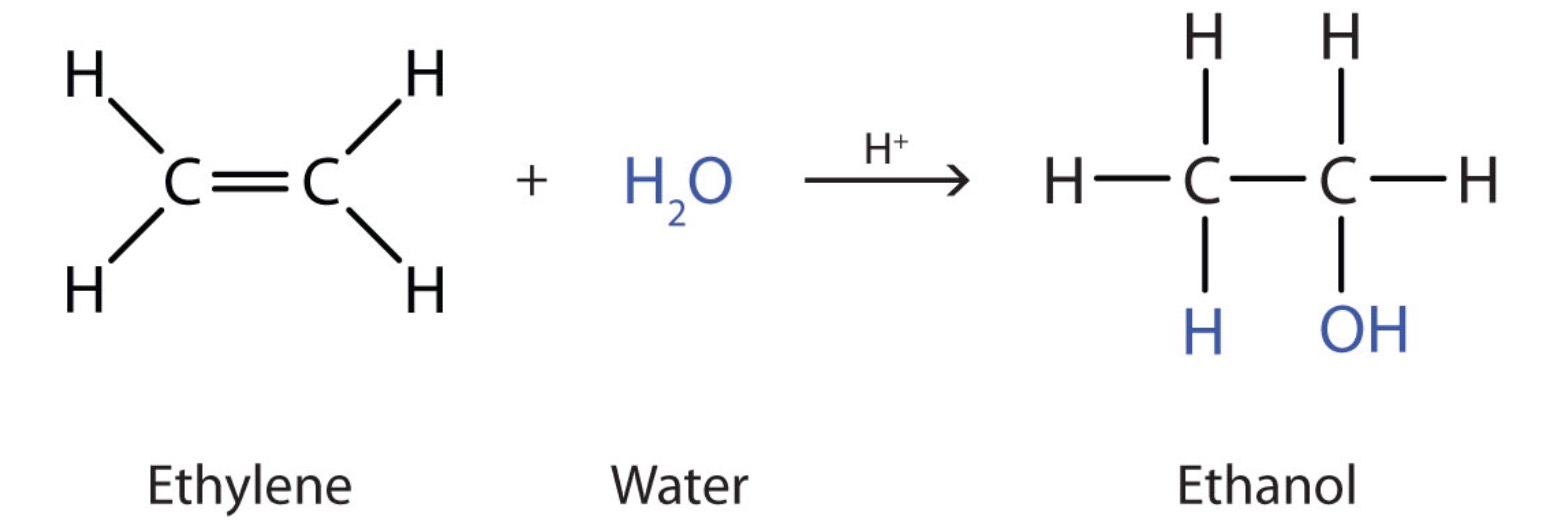
How can alkenes be used to make ethanol? Socratic
Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web 1 answer sorted by: Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the production of polymer. Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. Web water is.
Ethanol Metabolism Concise Medical Knowledge
Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain. Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the.
HYDROGEN BONDING YouTube
Methane is a gas, and so its. Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the production of polymer. Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. Web 1 answer sorted by: Web why doesn't methane dissolve in water?
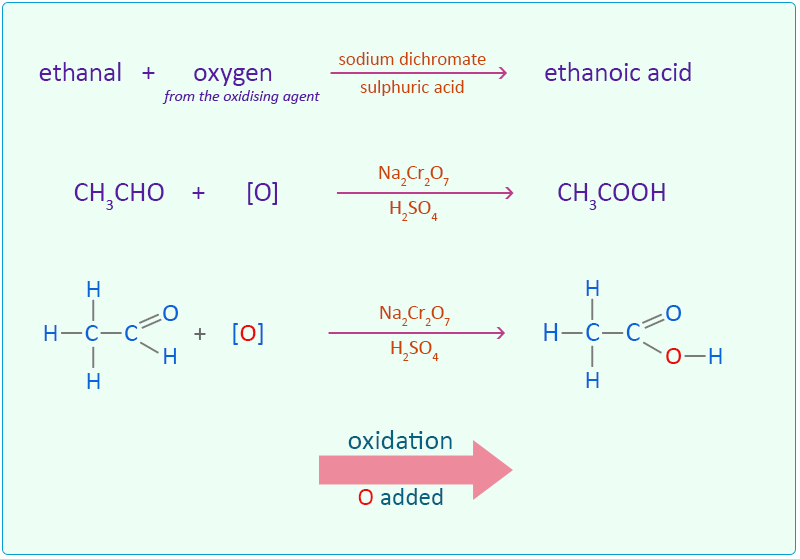
Oxidation of Ethanol Easy exam revision notes for GSCE Chemistry
Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the production of polymer. The methane, ch 4, itself is not the problem. Web this means the molecules will be soluble in a polar solvent such as water. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web 1 answer.
chemistry What are Hydrogen Bond Accepter and Hydrogen Bond Donor?
Web the chemical formula of ethane? Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that. Web 1 answer sorted by: Some examples of polar molecules which. All of the bonds between multiple water.
Hydrogen bond forms in liquid water as hydrogen atom of one molecule attracted towards the oxygen. Web 1 answer sorted by: Web intermolecular force of two polar water molecules forming a hydrogen bond. Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. 3 ethoxyethane, better known as diethyl ether or even just ether, can form hydrogen. The methane, ch 4, itself is not the problem. Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that. Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain. Web water is an even better example of a hydrogen bonding compound; All of the bonds between multiple water. What is the intramolecular bond that holds the hydrogen and carbon atoms within an. Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the production of polymer. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because. Web this means the molecules will be soluble in a polar solvent such as water. Web why doesn't methane dissolve in water? In fact, water molecules are so tightly bound to each. Some examples of polar molecules which. Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond.
Web Hydrogen Bonding Plays A Crucial Role In Many Biological Processes And Can Account For Many Natural Phenomena.
Web in water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that. Web water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. Hydrogen bond forms in liquid water as hydrogen atom of one molecule attracted towards the oxygen. Web as a result, water acts as a heat sink or heat reservoir and requires much more heat to boil than does a liquid such as ethanol (grain.
Web 1 Answer Sorted By:
Web water is an even better example of a hydrogen bonding compound; The methane, ch 4, itself is not the problem. Web ethane, {eq}\textrm{c}_2\textrm{h}_6 {/eq}, will not form hydrogen bonds with water, {eq}\textrm{h}_2\textrm{o} {/eq}, because. Web hydrogen bonding can occur between ethanol molecules, although not as effectively as in water.
Web Answer (1 Of 3):
Web for instance, it is an open question as to whether fluoroorganics can form a hydrogen bond. In fact, water molecules are so tightly bound to each. Web intermolecular force of two polar water molecules forming a hydrogen bond. Some examples of polar molecules which.
All Of The Bonds Between Multiple Water.
Web ethylene/ethane separation is validated by breakthrough experiments with high purity of ethylene (99.1%) at 333 k. Web this means the molecules will be soluble in a polar solvent such as water. Web why doesn't methane dissolve in water? Web the separation of ethane (c2h6) and ethylene (c2h4) represents a crucial process for the production of polymer.