What Is The Most Stable Monatomic Ion Formed From Aluminum - The aluminium belongs to group 13 of the periodic table. Web an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne] 3s2 3p1, [15] with three electrons beyond a stable noble gas configuration. The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web it is no longer used for this purpose because of the formation of the toxic gas phosgene, cl 2 co. The elements of this group have 3. Web currently, besides the trivalent aluminum ion, the alkali metals such as sodium and potassium (elia et al., 2016) and several. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion. Write the lewis structures for.
Ionic bonding
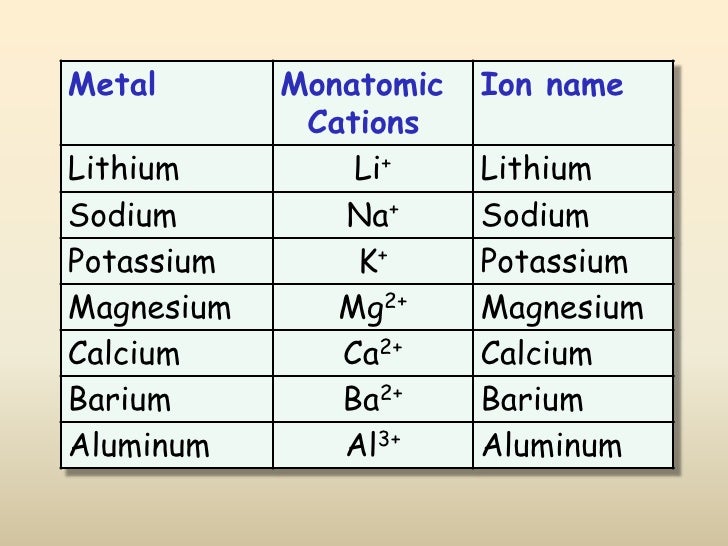
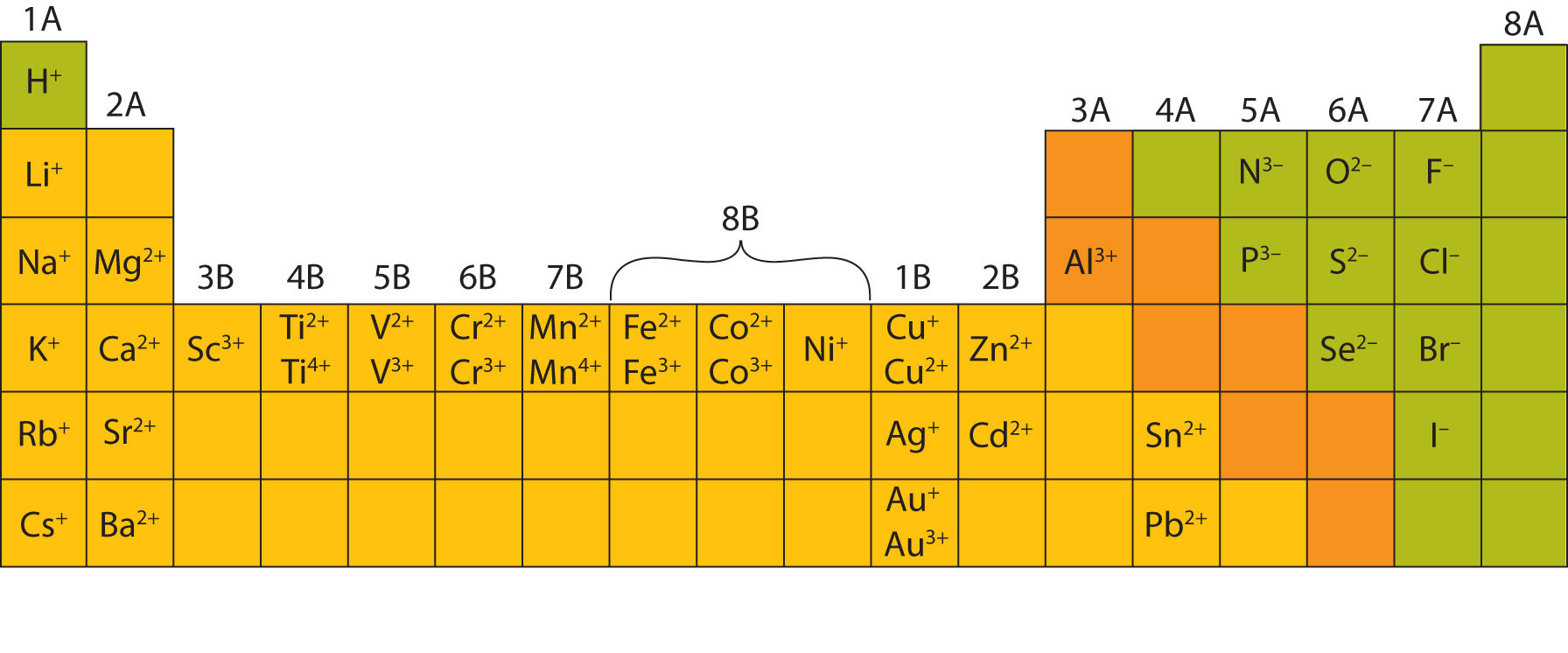
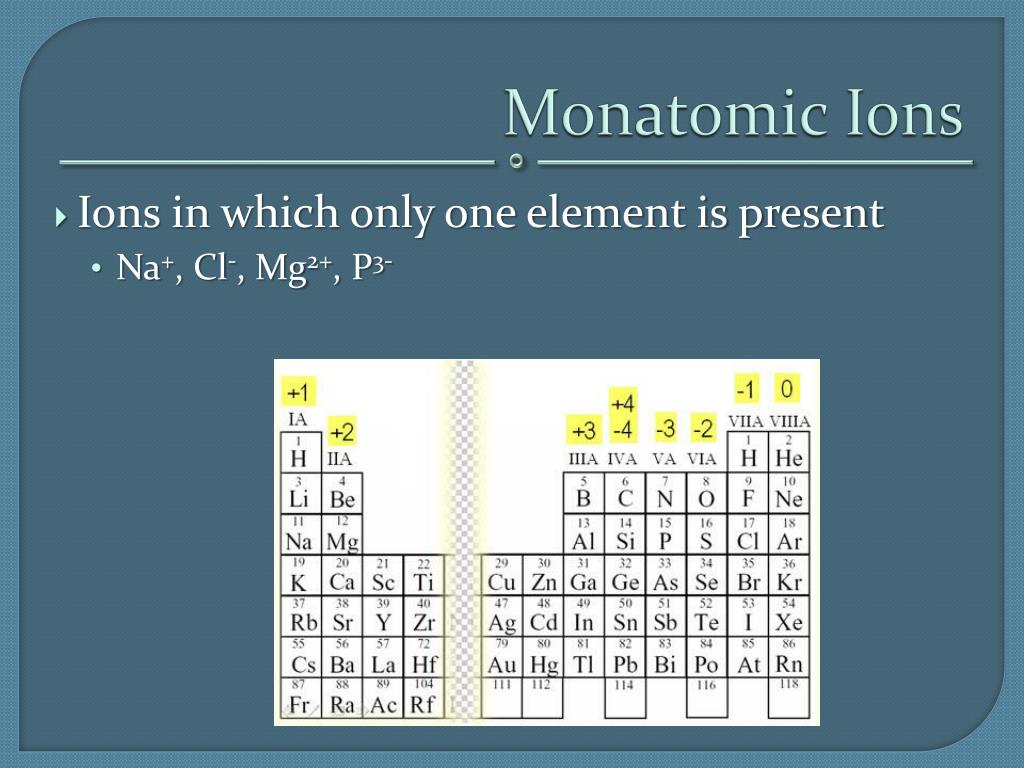
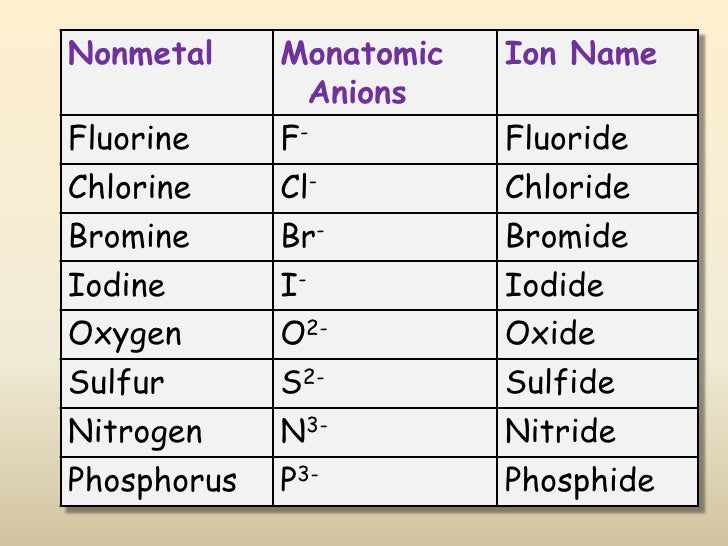
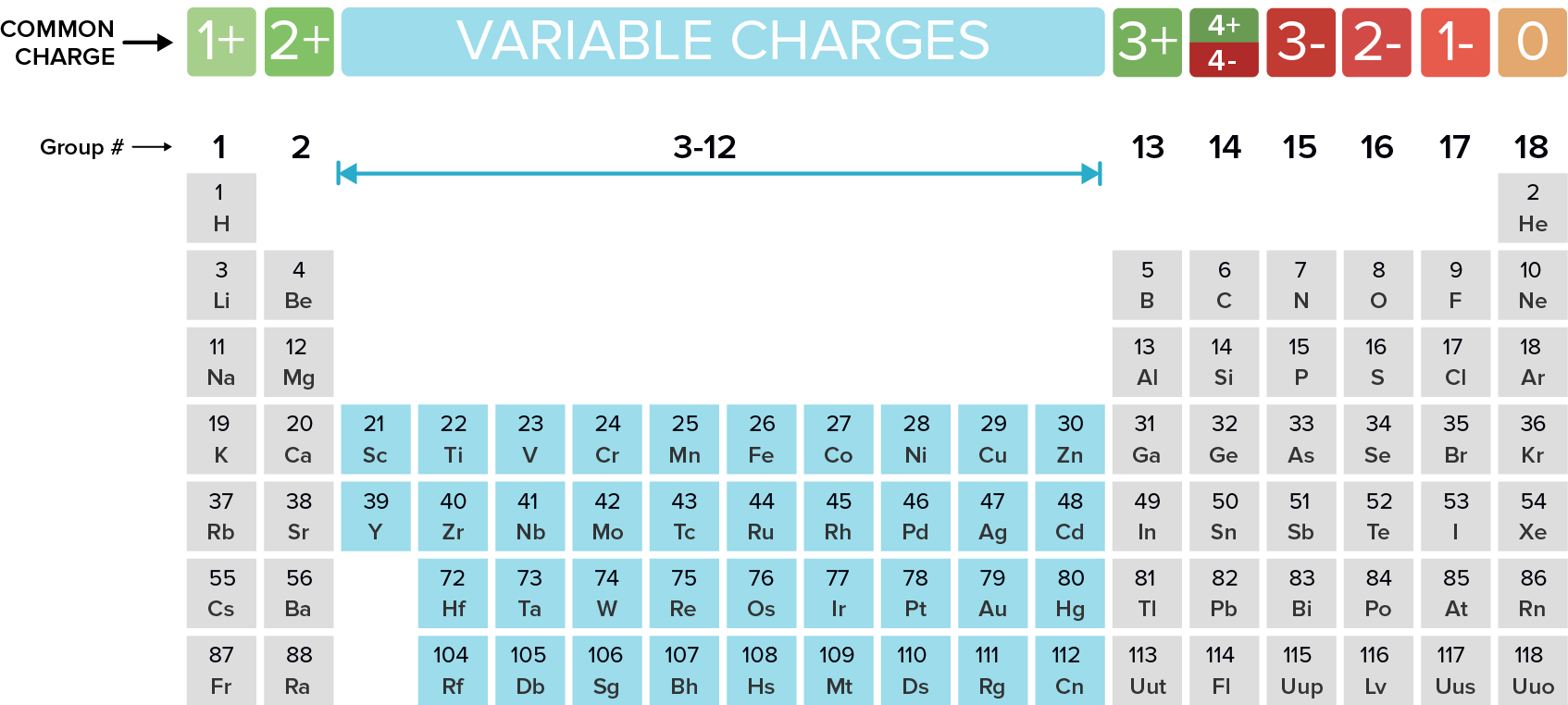
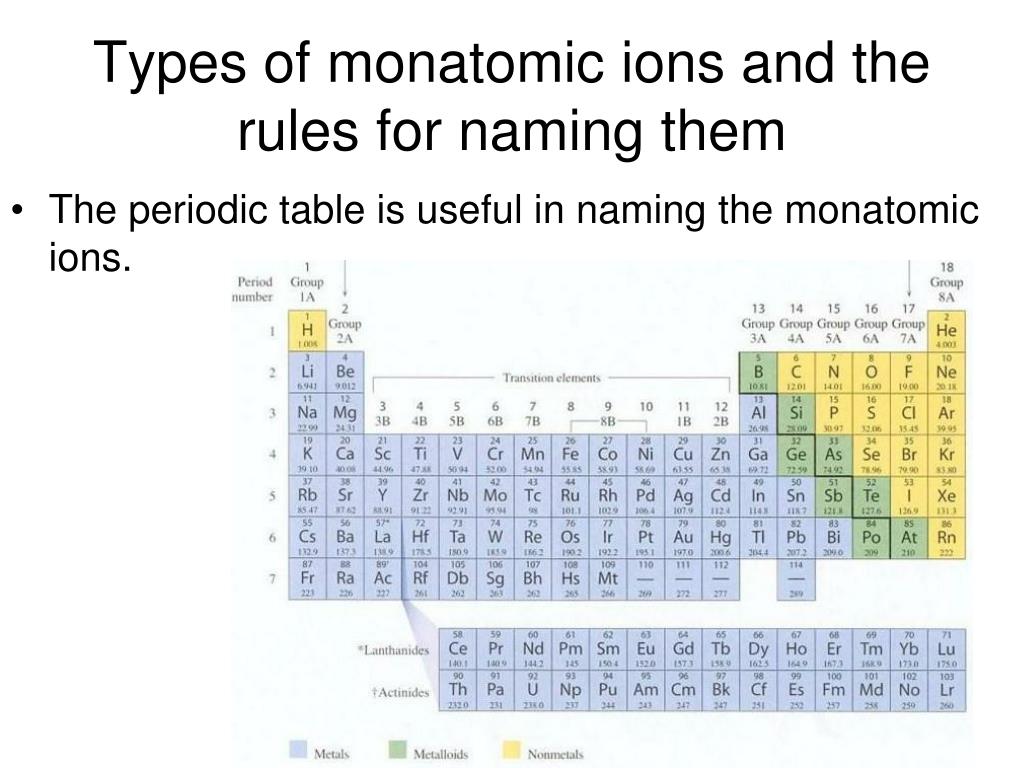
Web common type i monatomic cations hydrogen: 3s and 3p are the last orbitals that. The most stable ion is explanation: For example, fluorine will want 1 more electron to fill it's. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the.
Ions
Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Write the lewis structures for. Web common type i monatomic cations hydrogen: The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: Web what is the most stable monatomic ion formed from fluorine?
PPT Chapter 6 PowerPoint Presentation, free download ID3545809
The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: Web the aluminum ion of formula al 3+ has a defect of 3 electrons , to obtain its electronic structure there it is therefore sufficient to. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. 3s and 3p are the last orbitals.
PPT Ionic Compounds and Metals PowerPoint Presentation, free download
Mono atomic ion is defined. The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: Web common type i monatomic cations hydrogen: Aluminum is an element of group number 13 in the third period. Web currently, besides the trivalent aluminum ion, the alkali metals such as sodium and potassium (elia et al., 2016) and several.
Ionic bonding
Web al3+ as monatomic ion. The elements of this group have 3. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Web aluminum and carbon react to form an ionic compound. 3s and 3p are the last orbitals that.
Nomenclature Worksheet 1 Monatomic Ions Worksheet List
Web aluminum and carbon react to form an ionic compound. 3s and 3p are the last orbitals that. The aluminium belongs to group 13 of the periodic table. Write the lewis structures for. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable.
Ionic Naming
Web al3+ as monatomic ion. Write electron configurations for the most stable ion formed by each of the elements al, ba, se, and i (when in stable. The elements of this group have 3. Web the formula of the carbonate ion is co 3 2 −. Web the name of a binary compound containing monatomic ions consists of the name.
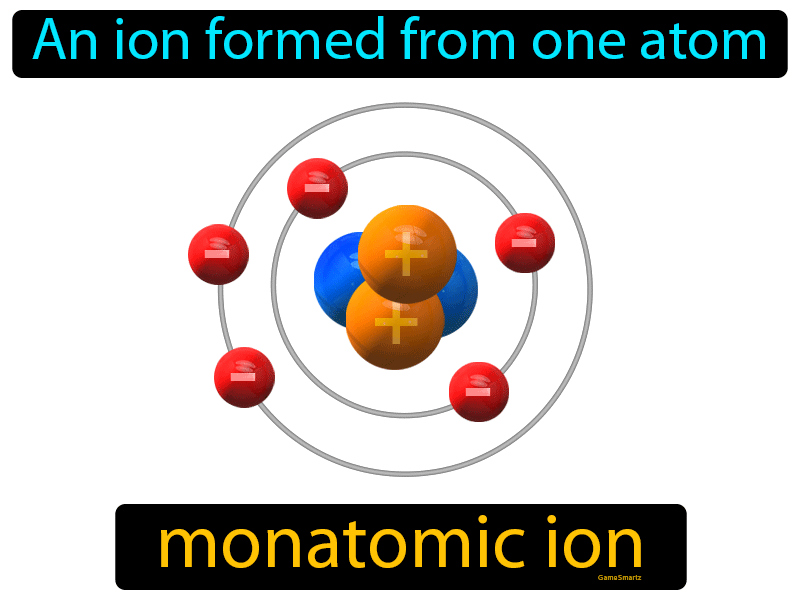
Monatomic Ion Definition Easy to Understand Game Smartz
Predict which forms an anion, which forms a cation, and the charges of. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion. Web.
PPT Monatomic Ions PowerPoint Presentation, free download ID3112818
3s and 3p are the last orbitals that. Web what is the most stable monatomic ion formed from fluorine? Web the formula of the carbonate ion is co 3 2 −. Web al3+ as monatomic ion. The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation:
PPT Monatomic Ions PowerPoint Presentation, free download ID3112818
Web aluminum and carbon react to form an ionic compound. Write the lewis structures for. The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: The most stable ion is explanation: The aluminium belongs to group 13 of the periodic table.
Web currently, besides the trivalent aluminum ion, the alkali metals such as sodium and potassium (elia et al., 2016) and several. The most stable ion is explanation: The elements of this group have 3. Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web the formula of the carbonate ion is co 3 2 −. Web the aluminum ion of formula al 3+ has a defect of 3 electrons , to obtain its electronic structure there it is therefore sufficient to. Aluminum is an element of group number 13 in the third period. Web common type i monatomic cations hydrogen: Web what is the most stable monatomic ion formed from fluorine? Predict which forms an anion, which forms a cation, and the charges of. The stable mono atomic ion of aluminium is [tex]al^{3+}[/tex] explanation: Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion. For example, fluorine will want 1 more electron to fill it's. Write electron configurations for the most stable ion formed by each of the elements al, ba, se, and i (when in stable. 3s and 3p are the last orbitals that. Web it is no longer used for this purpose because of the formation of the toxic gas phosgene, cl 2 co. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Write the lewis structures for. Web al3+ as monatomic ion. Web aluminum ions have a charge of 3+, as aluminum atoms lose three electrons to achieve the most stable electron.
Web Currently, Besides The Trivalent Aluminum Ion, The Alkali Metals Such As Sodium And Potassium (Elia Et Al., 2016) And Several.
3s and 3p are the last orbitals that. For example, fluorine will want 1 more electron to fill it's. Web aluminum and carbon react to form an ionic compound. Predict which forms an anion, which forms a cation, and the charges of.
The Elements Of This Group Have 3.
Web aluminum oxide is relatively unreactive because the small al 3 + ions and the o 2+ form a very stable ionic lattice in. Web the formula of the carbonate ion is co 3 2 −. Web the name of a binary compound containing monatomic ions consists of the name of the cation (the name of the metal) followed by the name of the anion. Web what is the most stable monatomic ion formed from fluorine?
The Most Stable Ion Is Explanation:
Web an aluminium atom has 13 electrons, arranged in an electron configuration of [ ne] 3s2 3p1, [15] with three electrons beyond a stable noble gas configuration. Web the aluminum ion of formula al 3+ has a defect of 3 electrons , to obtain its electronic structure there it is therefore sufficient to. Aluminum is an element of group number 13 in the third period. Mono atomic ion is defined.
Web It Is No Longer Used For This Purpose Because Of The Formation Of The Toxic Gas Phosgene, Cl 2 Co.
Write the lewis structures for. The atoms of a polyatomic ion are tightly bonded together and so the. Web aluminum ions have a charge of 3+, as aluminum atoms lose three electrons to achieve the most stable electron. Web common type i monatomic cations hydrogen: