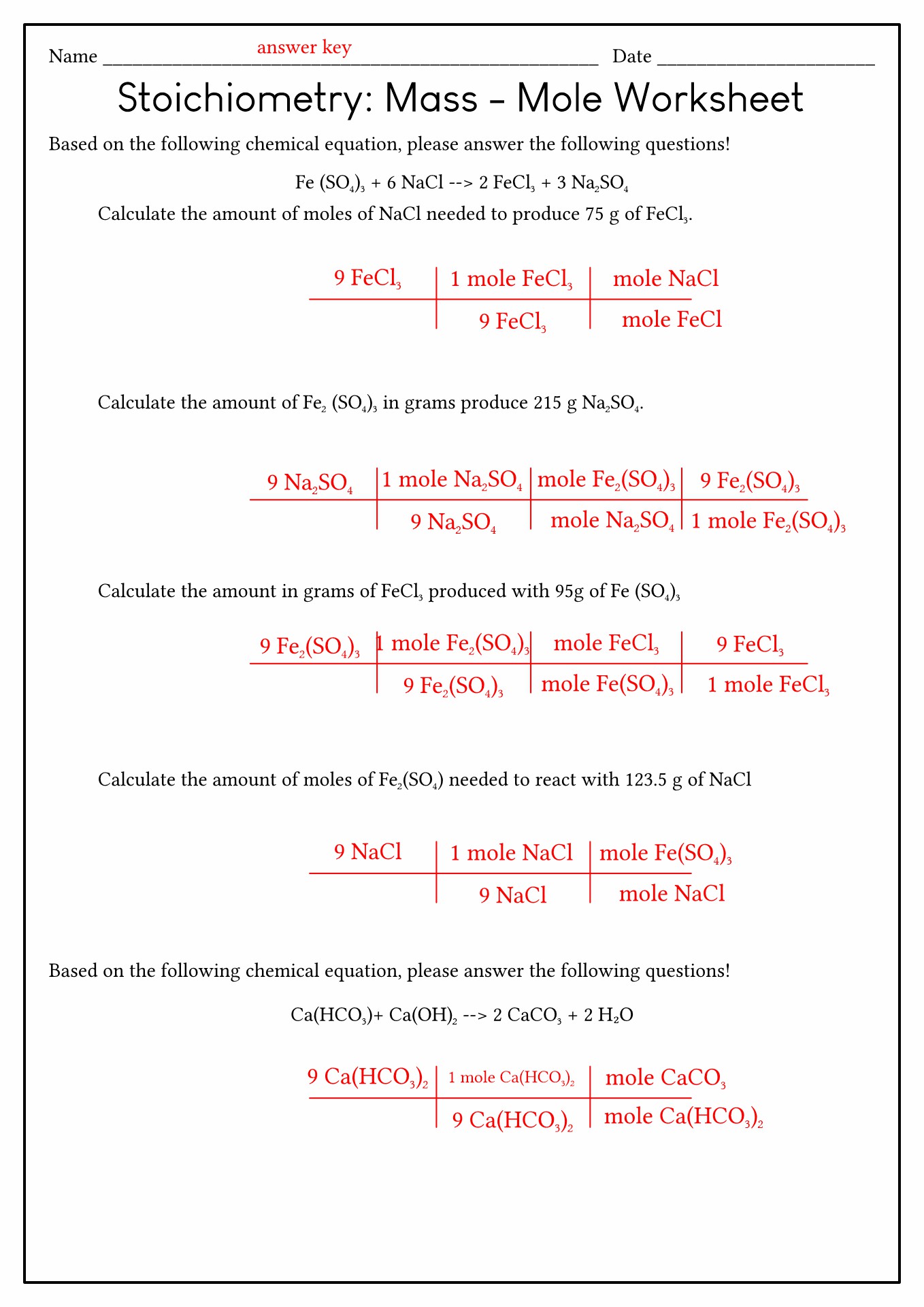
Stoichiometry Mole To Mole Problems Worksheet Answers - 10 / h 2o 2. N2 +3h2 name how many moles of hydrogen are needed to completely react with two. Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg. Web example #1:when 2.00 mol of n2reacts with sufficient h2, how many moles of nh3will be produced? Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. In this type of problem, the mass of one substance is given, usually in grams. Web mass to moles problems. How many moles of hydrogen are needed to completely react with 2 moles of nitrogen?. Web worksheet for basic stoichiometry part 1:
Image result for stoichiometry worksheet Persuasive writing prompts
Web mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium. Hydrogen gas can be produced through the following reaction. How many moles of hydrogen are needed to completely react with 2 moles of nitrogen?. N2 + 3h 2 → 2nh 3. Comments prior to solving the.
12 Best Images of Mole Ratio Worksheet Answer Key Mole Ratio
In this type of problem, the mass of one substance is given, usually in grams. 10 / h 2o 2. N2 +3h2 name how many moles of hydrogen are needed to completely react with two. N2 + 3h 2 → 2nh 3. Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +.
18 Best Images of Mole Conversion Problems Worksheet Answers Mole
Web mass to moles problems. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. Mole ←→ mass conversions convert the following number of moles of chemical. Hydrogen gas can be produced through the following reaction. Web mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium.
30 Worksheet Mole Problems Answers Education Template
Hydrogen gas can be produced through the following reaction. Mole to mass problems 1. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +. In this worksheet, students will start learning the concepts of.
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg. Web this stoichiometry practice worksheet covers mole ratios, mole to mole stoichiometry, limiting reactants, percent. Web worksheet for basic stoichiometry part 1: 10 / h 2o 2. Web these mass relationships, made through moles, are called stoichiometry (gk.
Mixed Mole stoichiometry worksheet
Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +. Web this is the first of four stoichiometry worksheets. Comments prior to solving the. In this type of problem, the mass of one substance is given, usually in grams. Web mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium.
Stoichiometry Worksheet+Answers Mole (Unit) Iron
Virginia state university (vsu) chemistry. Web this is the first of four stoichiometry worksheets. Web stoichiometry worksheet #1 answers 1. Web mass to moles problems. Web example #1:when 2.00 mol of n2reacts with sufficient h2, how many moles of nh3will be produced?
Mole Ratio Worksheet Doc Kiddo Worksheet
In this type of problem, the mass of one substance is given, usually in grams. Web this stoichiometry practice worksheet covers mole ratios, mole to mole stoichiometry, limiting reactants, percent. Web worksheet for basic stoichiometry part 1: How many moles of hydrogen are needed to completely react with 2 moles of nitrogen?. Hydrogen gas can be produced through the following.
FREE 9+ Sample Stoichiometry Worksheet Templates in MS Word PDF
Web this is the first of four stoichiometry worksheets. Web worksheet for basic stoichiometry part 1: Virginia state university (vsu) chemistry. Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg. Mole ←→ mass conversions convert the following number of moles of chemical.
Pin on Bored
Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular. Web mass to moles problems. 10 / h 2o 2. N2 + 3h 2 → 2nh 3. Web stoichiometry worksheet #1 answers 1.
Comments prior to solving the. 2141 grams 5 how many. Mole ←→ mass conversions convert the following number of moles of chemical. Virginia state university (vsu) chemistry. 10 / h 2o 2. Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg. How many moles of hydrogen are needed to completely react with 2 moles of nitrogen?. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular. N2 +3h2 name how many moles of hydrogen are needed to completely react with two. Web this stoichiometry practice worksheet covers mole ratios, mole to mole stoichiometry, limiting reactants, percent. Hydrogen gas can be produced through the following reaction. Web this is the first of four stoichiometry worksheets. Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +. Web mass to moles problems. N2 + 3h 2 → 2nh 3. In this type of problem, the mass of one substance is given, usually in grams. Web mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium. Web example #1:when 2.00 mol of n2reacts with sufficient h2, how many moles of nh3will be produced? 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. In this worksheet, students will start learning the concepts of.
N2 + 3H 2 → 2Nh 3.
Mole ←→ mass conversions convert the following number of moles of chemical. How many moles of hydrogen are needed to completely react with 2 moles of nitrogen?. Web this stoichiometry practice worksheet covers mole ratios, mole to mole stoichiometry, limiting reactants, percent. Web example #1:when 2.00 mol of n2reacts with sufficient h2, how many moles of nh3will be produced?
Web This Is The First Of Four Stoichiometry Worksheets.
N2 +3h2 name how many moles of hydrogen are needed to completely react with two. Derive an expression that relates the number of molecules in a sample of a substance to its mass and molecular. Web mass to moles problems. Virginia state university (vsu) chemistry.
Comments Prior To Solving The.
10 / h 2o 2. 40.08 grams of calcium is one mole (see periodic table), and one mole is 6.02 x 1023 atoms. Web stoichiometry worksheet #1 answers 1. Hydrogen gas can be produced through the following reaction.
In This Worksheet, Students Will Start Learning The Concepts Of.
Web mole calculation worksheet answer key 1 how many moles are in 15 grams of lithium. Web these mass relationships, made through moles, are called stoichiometry (gk stoicheon, element +. Mole to mass problems 1. Web the number of moles and the mass (in kg) of copper(ii) carbonate needed to decompose in order to produce 1.500 kg.