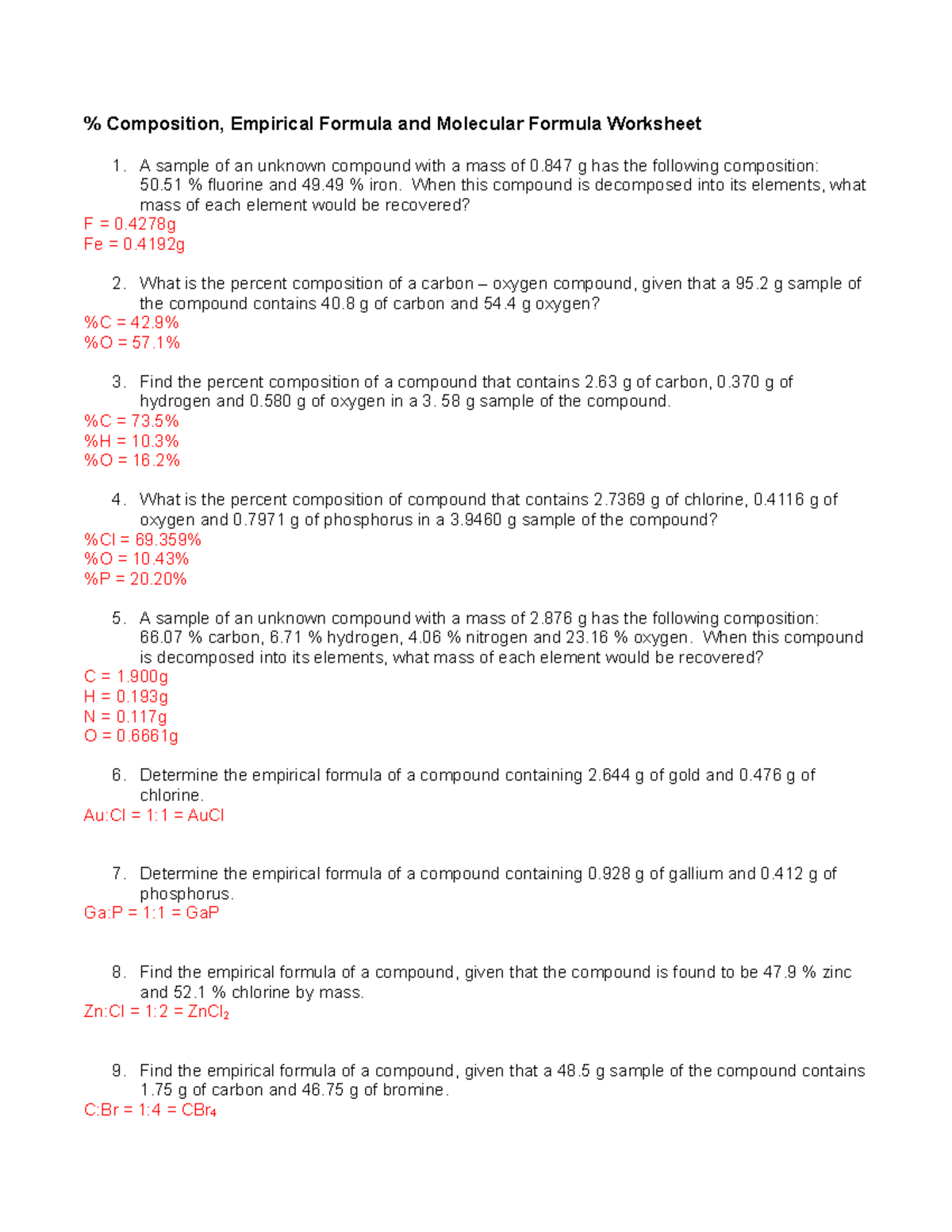
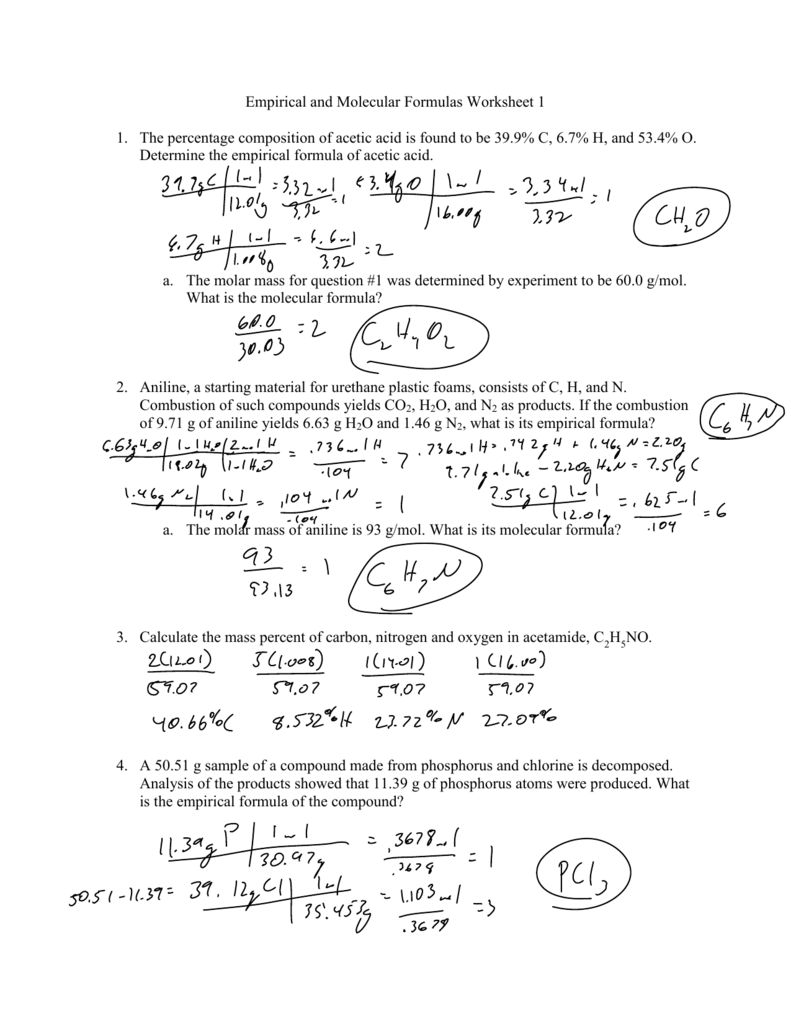
Percent Composition Empirical And Molecular Formulas Worksheet Answers - Percentage composition and empirical formulas. Empirical formulas work each of the following problems. To calculate percent composition, we divide the experimentally derived mass of each element by the. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Web practice calculating identifying and calculating empirical and molecular formulas, as well as two different types of. Find the empirical formula of a. A compound is found to contain 63.52 % iron. If the molar mass of the. Determine the empirical formulas for compounds with the following percent compositions: Exercise 4 a substance contains by mass 7.1% h,.
Mr. Zehner's Chemistry Class February 2011
A compound is found to contain 63.52 % iron. Web this is a package of two worksheets. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Web practice calculating identifying and calculating empirical and molecular formulas, as well as two different types of. Web what is the empirical formula?
percent composition and molecular formula worksheet key
The percentage composition of acetic acid is found to be. To calculate percent composition, we divide the experimentally derived mass of each element by the. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. A compound is found to contain 36.5% na, 25.4% s, and 38.1% o. Check answer/solution to exercise.
Empirical And Molecular Formulas Worksheet
Calculate the molecular or formula mass of each of the following: The percentage composition of acetic acid is found to be. Web what is the empirical formula? Web this is a package of two worksheets. The first worksheet gives your students practice with finding the percent composition of a.
worksheet. Percent Composition And Molecular Formula Worksheet. Grass
Determine the empirical formulas for compounds with the following percent compositions: The percentage composition of acetic acid is found to be. A compound is found to contain 63.52 % iron. Empirical formulas work each of the following problems. Determine the empirical formulas for compounds with the following percent compositions:
Chemical Formula Worksheet Answers
Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. To calculate percent composition, we divide the experimentally derived mass of each element by the. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of. The percentage composition of acetic.
Empirical and Molecular Formula Practice Problems
Web this is a package of two worksheets. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of. Calculate the molecular or formula mass of each of the following: Determine the empirical formulas for compounds with the following percent compositions: A compound is found to contain 36.5% na, 25.4% s, and 38.1%.
Percentage Composition and Empirical & Molecular Formula Worksheet for
Web this is a package of two worksheets. A compound is found to contain 63.52 % iron. Check answer/solution to exercise 3. The percentage composition of acetic acid is found to be. Empirical formulas work each of the following problems.
Lecture 10.3 (Honors only) Percent Composition & Empirical Formulas
Web 1.calculate the mass of the empirical formula (which you have already found or it will be given to you ) 2. Exercise 4 a substance contains by mass 7.1% h,. Empirical formulas work each of the following problems. Check answer/solution to exercise 3. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical.
Chemistry Unit 4 Worksheet 2 Answers
Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of. A compound is found to contain 36.5% na, 25.4% s, and 38.1% o. Web what is the empirical formula? Percentage composition and empirical formulas. Web this is a package of two worksheets.
Percent Composition Empirical And Molecular Formulas Worksheet Answers
Exercise 4 a substance contains by mass 7.1% h,. To calculate percent composition, we divide the experimentally derived mass of each element by the. Web practice calculating identifying and calculating empirical and molecular formulas, as well as two different types of. The percentage composition of acetic acid is found to be. The first worksheet gives your students practice with finding.
If the molar mass of the. Calculate the molecular or formula mass of each of the following: A compound is found to contain 63.52 % iron. Check answer/solution to exercise 3. A compound is found to contain 63.52 % iron. Web 1.calculate the mass of the empirical formula (which you have already found or it will be given to you ) 2. To calculate percent composition, we divide the experimentally derived mass of each element by the. Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole. Web this is a package of two worksheets. Exercise 4 a substance contains by mass 7.1% h,. Percentage composition and empirical formulas. Find the empirical formula of a. To calculate percent composition, we divide the experimentally derived mass of each element by the overall. Determine the empirical formulas for compounds with the following percent compositions: The percentage composition of acetic acid is found to be. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no. Empirical formulas work each of the following problems. What’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of.
Web This Is A Package Of Two Worksheets.
Determine the empirical formulas for compounds with the following percent compositions: The percentage composition of acetic acid is found to be. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of. Check answer/solution to exercise 3.
Web 1.Calculate The Mass Of The Empirical Formula (Which You Have Already Found Or It Will Be Given To You ) 2.
Empirical formulas work each of the following problems. A compound is found to contain 36.5% na, 25.4% s, and 38.1% o. If the molar mass of the. Web a compound with an empirical formula of c 2 h 8 n and a molar mass of 46 grams per mole.
To Calculate Percent Composition, We Divide The Experimentally Derived Mass Of Each Element By The.
Determine the empirical formulas for compounds with the following percent compositions: Calculate the molecular or formula mass of each of the following: A compound is found to contain 63.52 % iron. Percent composition of c = 65.5%, h = 5.5%, o = 29.0% for determining the empirical formula, no.
To Calculate Percent Composition, We Divide The Experimentally Derived Mass Of Each Element By The.
Empirical formulas work each of the following problems. To calculate percent composition, we divide the experimentally derived mass of each element by the overall. Web what is the empirical formula? Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a.