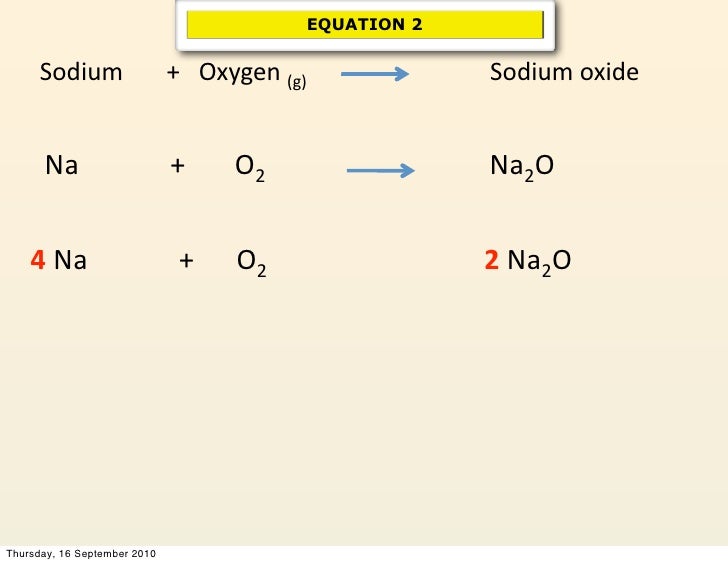
Sodium Oxide Combines With Water To Form Sodium Hydroxide - It fizzes rapidly before it. Sodium oxide and water combine to form sodium hydroxide. Web chemical properties generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base,. Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Na2o + h2o → 2naoh b. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Na2o + h2o → 2nah + o2 c. So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can. Web what is the balanced equation for sodium oxide combines with water to make sodium hydroxide? Web sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution.
Balanced Equation For Sodium Water Gives Hydroxide Hydrogen Tessshebaylo
Sodium oxide and water combine to form sodium hydroxide. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. In the given reaction, sodium reacts with water vigorously to form sodium hydroxide and. Yes it is a hydroxide for a word equasion it is sodium. Web sodium oxide reacts with water to produce sodium hydroxide.
Kb values of naoh
Web when sodium is added to water, the sodium melts to form a ball that moves around on the surface. Yes it is a hydroxide for a word equasion it is sodium. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Web in other words, sodium will readily reduce water, but zinc will not. 20.0 g of sodium.
PPT Each reaction will usually be worth a total of 5 points
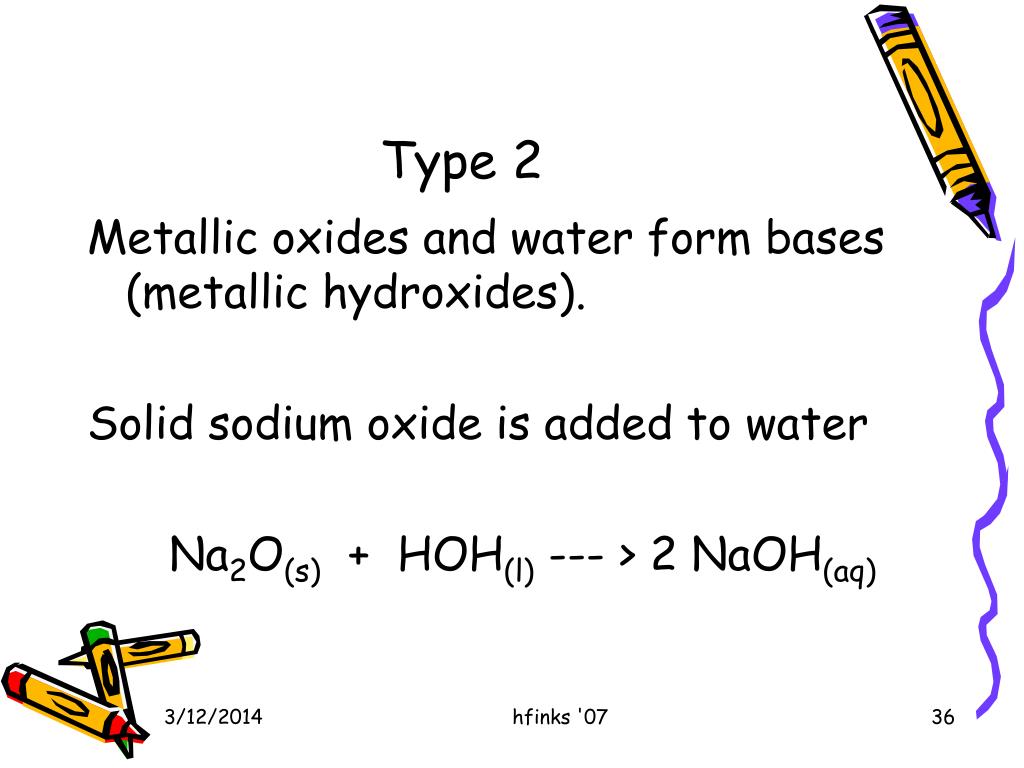
Web sodium oxide reacts with water to produce sodium hydroxide. Web sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. Web sodium oxide combines with water to form sodium hydroxide? Web the balanced equation for the reaction between sodium oxide and water is a. Web can you please balance this equation for me:
Chemical Equation Between Sodium Oxide And Water Tessshebaylo
Web reaction with water: Web sodium oxide reacts with water to produce sodium hydroxide. Web reaction information word equation sodium + water = sodium hydroxide + dihydrogen two moles of sodium [na] and two moles. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Since zinc cannot directly reduce water, it will.
Balance Chemical Equation For Sodium Water Gives Hydroxide Hydrogen
Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Web reaction information word equation sodium + water = sodium hydroxide + dihydrogen two moles of sodium [na] and two moles. Web can you please balance this equation for me: Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide. Web sodium oxide reacts exothermically with cold.
savvychemist GCSE OCR Gateway Chemistry C2.2 di Bonding and the
Web can you please balance this equation for me: Web reaction with water: In the given reaction, sodium reacts with water vigorously to form sodium hydroxide and. Sodium oxide combines with water to form sodium. Web sodium oxide reacts with water to produce sodium hydroxide.
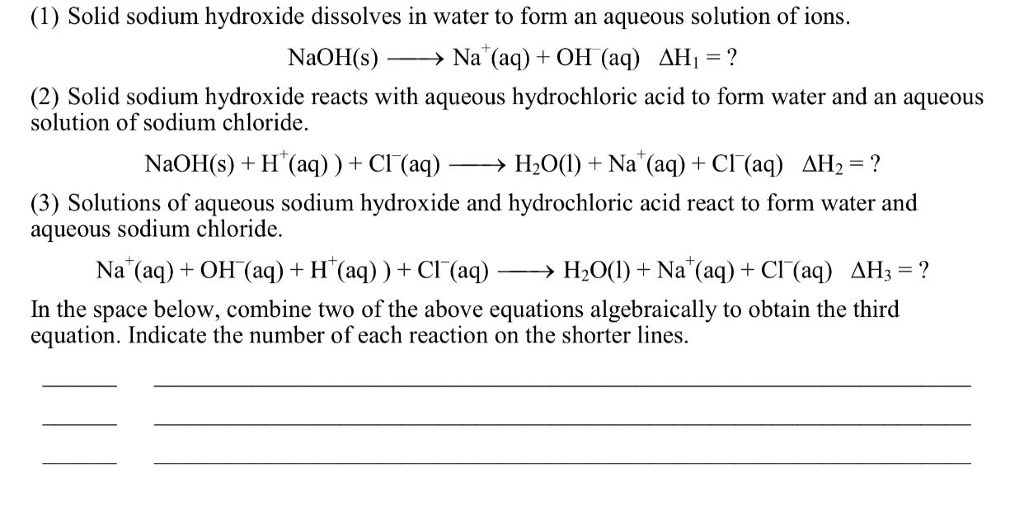
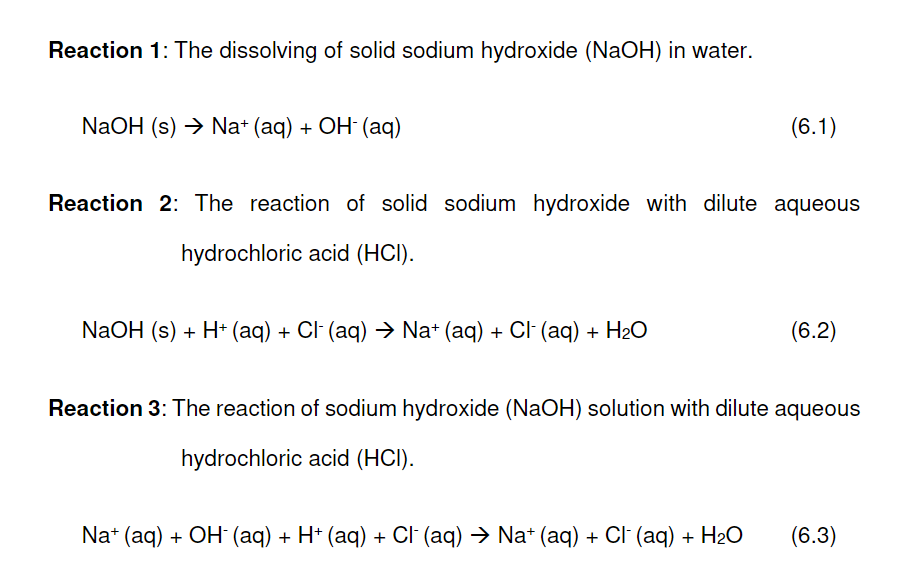
Solved Reaction 1 The dissolving of solid sodium hydroxide
Web can you please balance this equation for me: 20.0 g of sodium oxide is dissolved in 500 ml of water. Na2o + h2o → 2naoh b. Na2o + h2o → 2nah + o2 c. The chemical equation for this reaction is given.
PPT Chapter 5 A Closer Look at Chemical Equations PowerPoint
Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Web reaction information word equation sodium + water = sodium hydroxide + dihydrogen two moles of sodium [na] and two moles. Web sodium oxide combines with water to form sodium hydroxide? Web expert answer transcribed image text: The chemical equation for this reaction is given.
Chemical Reactions
Web the balanced equation for the reaction between sodium oxide and water is a. Web viewed 143k times. Web sodium oxide, na 2 o, has been well studied since last century and widely used in pharmaceutics, ceramics, and. Web sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. In the given reaction, sodium.
PPT Balancing Equations ANSWER KEY PowerPoint Presentation ID2276630
Web sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. Web when sodium is added to water, the sodium melts to form a ball that moves around on the surface. The chemical equation for this reaction is given. Web in other words, sodium will readily reduce water, but zinc will not. Web reaction.
Web expert answer transcribed image text: It fizzes rapidly before it. Web what is the balanced equation for sodium oxide combines with water to make sodium hydroxide? So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can. Web can you please balance this equation for me: Web sodium oxide, na 2 o, has been well studied since last century and widely used in pharmaceutics, ceramics, and. Web word equation to symbolic equation: Web sodium oxide reacts with water to produce sodium hydroxide. Web in other words, sodium will readily reduce water, but zinc will not. 20.0 g of sodium oxide is dissolved in 500 ml of water. Web sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. Na2o + h2o → 2naoh b. Sodium oxide and water combine to form sodium hydroxide. Web chemical properties generally, elemental sodium is more reactive than lithium, and it reacts with water to form a strong base,. Write the word equation and a balanced chemical equation for each. Sodium oxide combines with water to form sodium. In the given reaction, sodium reacts with water vigorously to form sodium hydroxide and. Web the balanced equation for the reaction between sodium oxide and water is a. Web sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. The chemical equation for this reaction is given.
Web What Is The Balanced Equation For Sodium Oxide Combines With Water To Make Sodium Hydroxide?
Web can you please balance this equation for me: So i wanted to know what the reaction between sodium hydroxide and carbon dioxide can. Web expert answer transcribed image text: Web viewed 143k times.
Since Zinc Cannot Directly Reduce Water, It Will.
Web if sodium reacts with an excess of water at standard conditions, dissolved sodium hydroxide and hydrogen. Na2o + h2o → 2nah + o2 c. 20.0 g of sodium oxide is dissolved in 500 ml of water. Na+h2o>naoh that is sodium reacting with water to give sodium hydroxide.
It Fizzes Rapidly Before It.
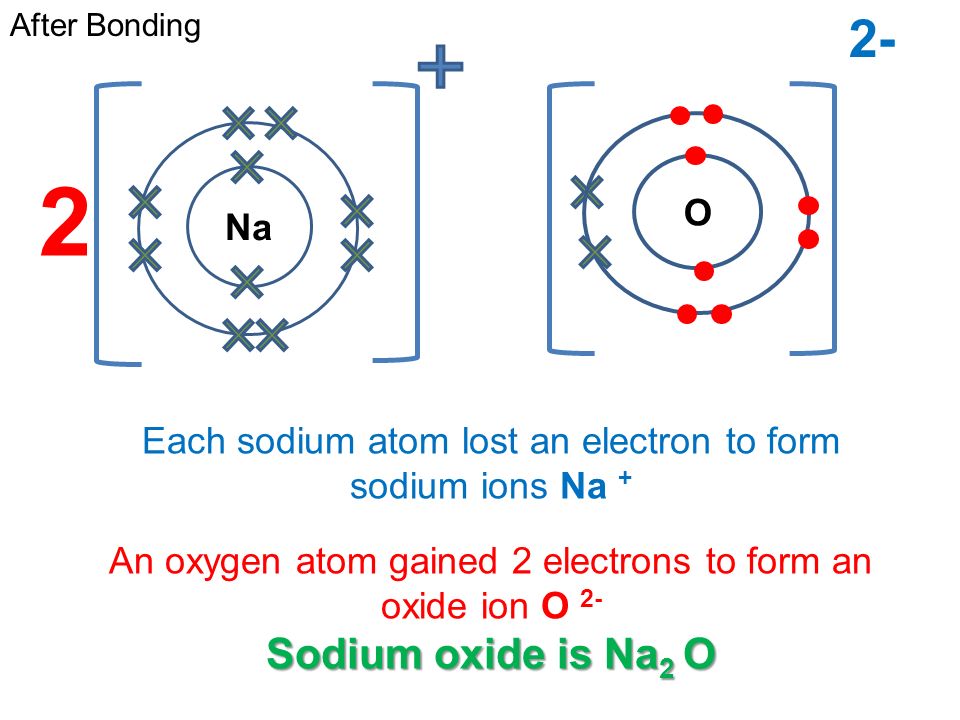
Sodium oxide combines with water to form sodium. Web when sodium is added to water, the sodium melts to form a ball that moves around on the surface. Yes it is a hydroxide for a word equasion it is sodium. Web word equation to symbolic equation:
Web Sodium Oxide Reacts Exothermically With Cold Water To Produce Sodium Hydroxide Solution.
Sodium oxide and water combine to form sodium hydroxide. Web sodium oxide reacts with some acids (such as hydrochloric acid) to form sodium chloride and water. Web in other words, sodium will readily reduce water, but zinc will not. Web sodium oxide, na 2 o, has been well studied since last century and widely used in pharmaceutics, ceramics, and.