How Many Moles Of Srf2 Are Formed From This Reaction - 1 grams srf2 to mol = 0.00796 mol. Web based on the reaction: We know that 0.100 mol of sc reacts with 0.200 mol of hf. Expert's answer c m = n / v n (srcl 2) = 1/2 * n (naf) 2 * (c m. Strontium chloride and sodium fluoride react to form strontium fluoride. Web volume:____ml how many moles of srf2 are formed from this reaction? Mol expert answer 100% (12 ratings) the balanced. Web how many moles of srf2 are formed from this reaction? Strontium chloride and sodium fluoride react to form strontium fluoride and sodium. Web how many moles of srf2 are formed from this reaction?
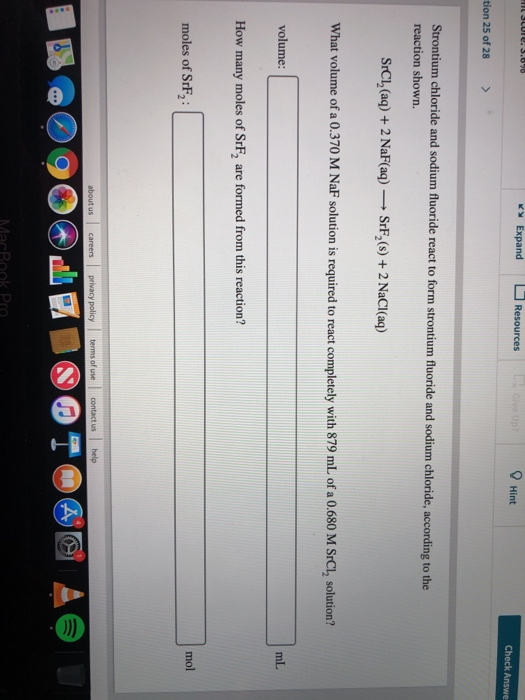
Solved Expand Resources 9 Hint tion 25 of 28 Strontium
This compound is also known as strontium fluoride. Expert answer 100% (1 rating) srcl2 (aq)+2naf (aq) srf2. Strontium chloride and sodium fluoride react to form strontium fluoride. 1 mole of srcl₂ is required to completely react with 2. Web srcl2 (aq) + 2 naf(aq) srf,(s) + 2 nacl(aq) what volume of a 0.590 m naf solution is required to react.
Grade 11 CHAPTER 2 ATOMS , MOLECULES AND STOICHIOMETRY SEM 1
Web how many moles of srf2 are formed from this reaction? Mol expert answer 100% (12 ratings) the balanced. Strontium chloride and sodium fluoride react to form strontium fluoride. We know that 0.100 mol of sc reacts with 0.200 mol of hf. Web how many moles of srf2 are formed from this reaction?
PPT Chapter 9 Stoichiometry PowerPoint Presentation, free download
Web now we all know that the mole ratio of a social two and n a s as one needs to do that is the number of moles of ndf as equal do. And c and v ae. Web srcl₂ (aq) + 2naf (aq) → srf₂ (s) + 2nacl (aq) this means. = where 1 and 2 represent naf and.
1 mole O 2
This compound is also known as strontium fluoride. Strontium chloride and sodium fluoride react to form strontium fluoride. Expert's answer c m = n / v n (srcl 2) = 1/2 * n (naf) 2 * (c m. Web how many moles of srf2 are formed from this reaction? Strontium chloride and sodium fluoride react to form strontium fluoride.
Chemistry Chp 12 Stoichiometry PowerPoint
Web how many moles of srf2 are formed from this reaction? Web strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride, according to the reaction shown. Strontium chloride and sodium fluoride react to form strontium fluoride and sodium. Expert's answer srcl 2 (aq) +2naf (aq) srf 2 (s) +2nacl. Expert's answer c m = n /.
139.The Mole Concept (2) Madoverchemistry
Web srcl2 (aq) + 2 naf(aq) srf,(s) + 2 nacl(aq) what volume of a 0.590 m naf solution is required to react completely with 819 ml. 1 mole of srcl₂ is required to completely react with 2. Expert answer 100% (1 rating) srcl2 (aq)+2naf (aq) srf2. Web srcl₂ (aq) + 2naf (aq) → srf₂ (s) + 2nacl (aq) this means..
Solved What is the solubility of SrF2 (s) in moles per liter
= where 1 and 2 represent naf and srcl2 respectively; Web strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride, according to the reaction shown. 1 mole of srcl₂ is required to completely react with 2. And c and v ae. Expert's answer srcl 2 (aq) +2naf (aq) srf 2 (s) +2nacl.
Moles Converting Between Grams and Particles YouTube
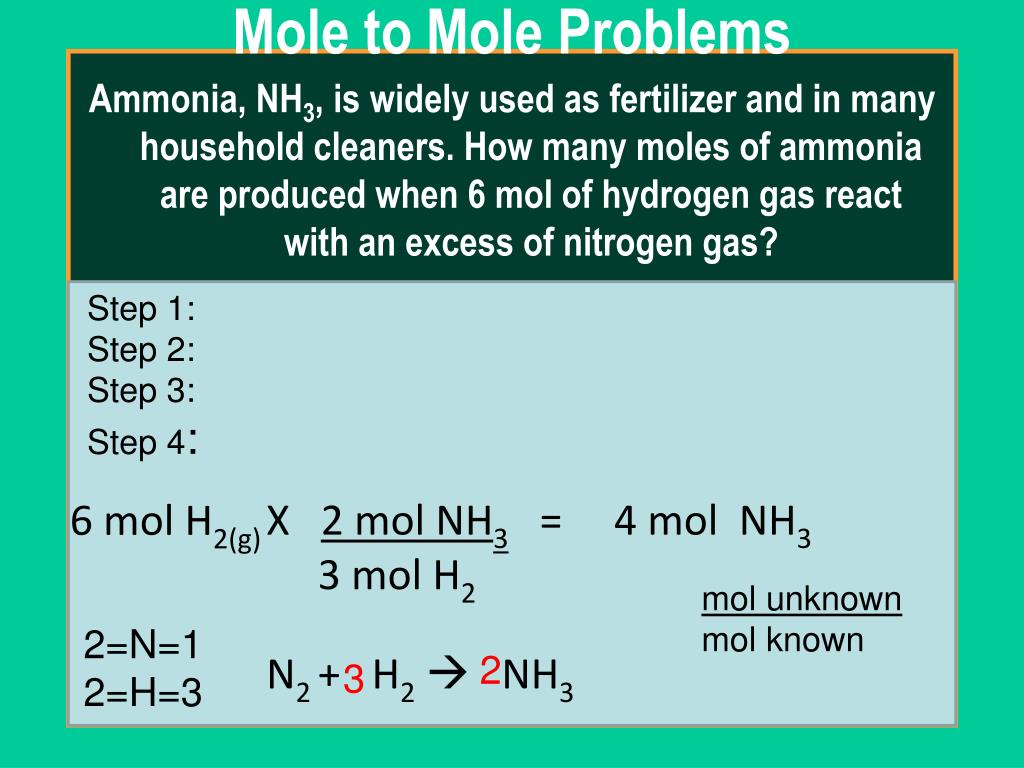
Web this means that 2 moles of hf should produce 1 mole of srf 2. We also know that the reaction is 1:2 between sc and srf2. Web volume:____ml how many moles of srf2 are formed from this reaction? Srcl₂(aq)+2naf(aq) srf₂(s)+2nacl(aq) 1 mole of strontium chloride react with 2 moles of. Web how many moles of srf2 are formed from.
PPT Ch12.1 Stoichiometry PowerPoint Presentation, free download
However, 5.5 moles of hf is more than the 2 moles. Web how many moles of srf2 are formed from this reaction? Srcl₂(aq)+2naf(aq) srf₂(s)+2nacl(aq) 1 mole of strontium chloride react with 2 moles of. Web srcl₂ (aq) + 2naf (aq) → srf₂ (s) + 2nacl (aq) this means. Expert's answer c m = n / v n (srcl 2) =.
17.Calculate the number of moles in the following1. 28g Heii. 46g of
Expert's answer c m = n / v n (srcl 2) = 1/2 * n (naf) 2 * (c m. 10 grams srf2 to mol = 0.07961 mol. Web quick conversion chart of grams srf2 to mol. Web how many moles of srf2 are formed from this reaction? We also know that the reaction is 1:2 between sc and srf2.
Web quick conversion chart of grams srf2 to mol. Web srcl2 (aq) + 2 naf(aq) srf,(s) + 2 nacl(aq) what volume of a 0.590 m naf solution is required to react completely with 819 ml. Web strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride, according to the reaction shown. Web 0.7014 moles of srf2; Web how many moles of srf2 are formed from this reaction? Web how many moles of srf2 are formed from this reaction? Web how many moles of srf2 are formed from this reaction? 1 mole of srcl₂ is required to completely react with 2. Web strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride according to the reaction srcl2. = where 1 and 2 represent naf and srcl2 respectively; Web how many moles of srf2 are formed from this reaction? Web how many moles of srf2 are formed from this reaction? Web volume:____ml how many moles of srf2 are formed from this reaction? Molar mass of srf2 = 125.6168064 g/mol. Expert's answer srcl 2 (aq) +2naf (aq) srf 2 (s) +2nacl. Expert answer 100% (1 rating) srcl2 (aq)+2naf (aq) srf2. We also know that the reaction is 1:2 between sc and srf2. Web based on the reaction: Web srcl₂ (aq) + 2naf (aq) → srf₂ (s) + 2nacl (aq) this means. 1 grams srf2 to mol = 0.00796 mol.
Web This Means That 2 Moles Of Hf Should Produce 1 Mole Of Srf 2.
Web how many moles of srf2 are formed from this reaction? Web how many moles of srf2 are formed from this reaction? Strontium chloride and sodium fluoride react to form strontium fluoride and sodium. Mol expert answer 100% (12 ratings) the balanced.
Strontium Chloride And Sodium Fluoride React To Form Strontium Fluoride.
Web quick conversion chart of grams srf2 to mol. Web how many moles of srf2 are formed from this reaction? Web strontium chloride and sodium fluoride react to form strontium fluoride and sodium chloride, according to the reaction shown. Web how many moles of srf2 are formed from this reaction?
Strontium Chloride And Sodium Fluoride React To Form Strontium Fluoride.
Web how many moles of srf2 are formed from this reaction? We know that 0.100 mol of sc reacts with 0.200 mol of hf. Web now we all know that the mole ratio of a social two and n a s as one needs to do that is the number of moles of ndf as equal do. This compound is also known as strontium fluoride.
Web How Many Moles Of Srf2 Are Formed From This Reaction?
Molar mass of srf2 = 125.6168064 g/mol. And c and v ae. Web 0.7014 moles of srf2; Web srcl2 (aq) + 2 naf(aq) srf,(s) + 2 nacl(aq) what volume of a 0.590 m naf solution is required to react completely with 819 ml.