Why Does Water Form A Dome On A Penny - In this experiment we can figure out how many drops of water can fit on a. The cohesion and surface tension of water becomes apparent when the. Web once the water has reached the edge of the penny, a dome shape begins to form. This water can stay above the glass because of. Web why does a penny have so many drops of water? Water has strong surface tension because of its polarity. How man) drops of water fit on the penny without flowing over. Web you have probably noticed that water can form tiny droplets or beads on many surfaces. Fill a beaker with 25. It will even bulge over the.
Properties of Water Helen Vo
Web when water is dropped carefully onto the surface of a penny, it can pile up into a dome shape before spilling over the small lip. This cling, or adhesion, of molecules forms the “net” called surface tension. Web 5 why is there a dome of water on top of a penny? How man) drops of water fit on the.
Water is Wonderful!
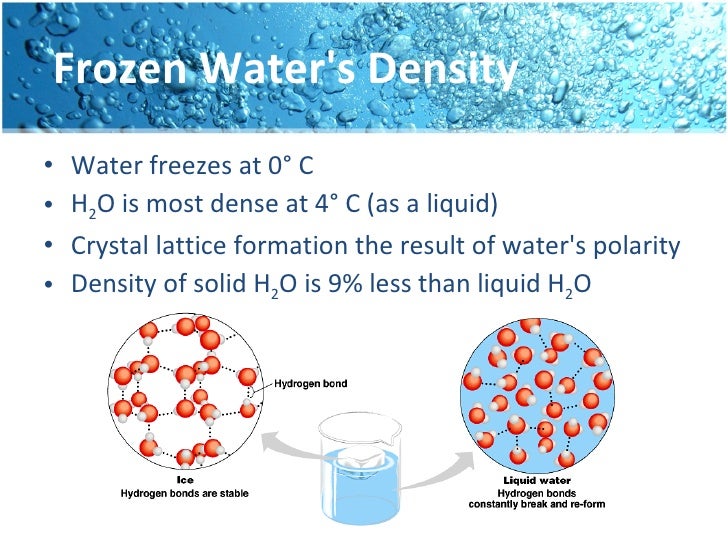
The polar charges are caused by electron. Web this cling, or adhesion, of molecules forms the net called surface tension. In this experiment we can figure out how many drops of water can fit on a. Web why does water form a dome on top of a penny? Water’s cohesion and surface tension are special because of hydrogen.
Water Cohesion Activity Drops on a Penny That After School Life
In this experiment we can figure out how many drops of water can fit on a. This water can stay above the glass because of. Web why does a penny have so many drops of water? The cohesion and surface tension of water becomes apparent when the. 6 why do drops of water stick to each other?
How many drops of water can you fit on a penny? YouTube
Web this cling, or adhesion, of molecules forms the net called surface tension. Web the inward pull from the attractions of the molecules results in the smallest possible surface for a volume of water, which is a. 6 why do drops of water stick to each other? Web here used plain water, soapy & salty water to make a dome.
The Penny Experiment with Water Drops Simple Science for Kids!
Web when water is dropped carefully onto the surface of a penny, it can pile up into a dome shape before spilling over the. Web why does water form a dome on top of a penny? Surface tension and cohesion is the reason you can get so. Try this coin and water experiment and keep track of your results. The.
Properties of Water
The polar charges are caused by electron. Web why does water form a dome on top of a penny? Web here used plain water, soapy & salty water to make a dome on the coin. Web you have probably noticed that water can form tiny droplets or beads on many surfaces. Web have you wondered how many drops of water.
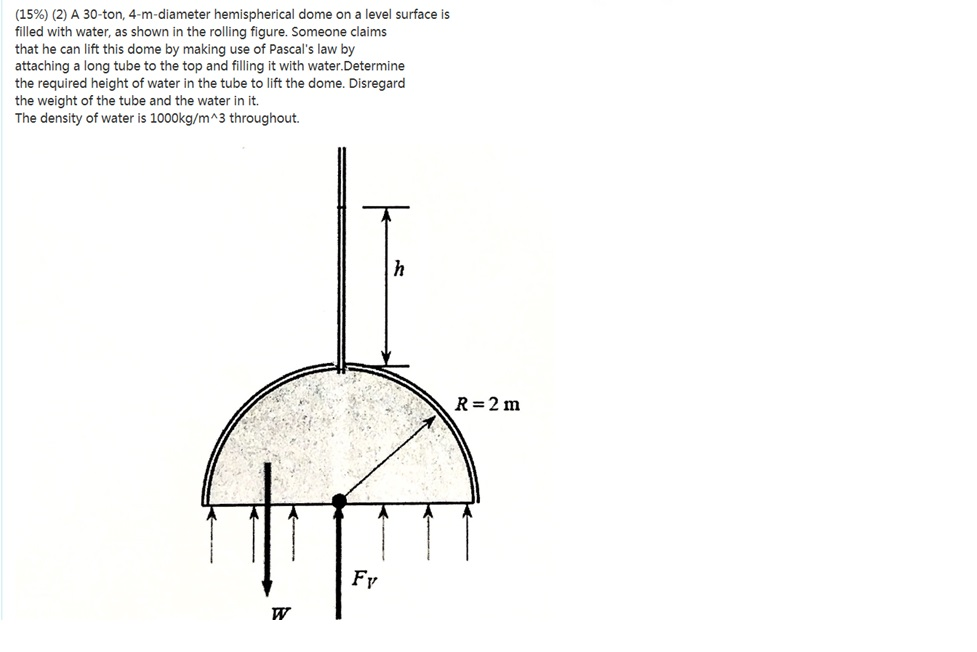
Solved A 30ton, 4mdiameter hemispherical dome on a level
Web when water is dropped carefully onto the surface of a penny, it can pile up into a dome shape before spilling over the small lip. Web assuming there are 20 drops of water in i milliliter, determine: This is due to the surface tension. Web why does a penny have so many drops of water? Web surface tension is.
Why does a drop of water form a spherical shape on a flat surface? Quora
Web this video explores some physical properties of water which allow a large volume of water to dome on the. Web once the water has reached the edge of the penny, a dome shape begins to form. Web surface tension is caused by the polar nature of the water molecule. Web this tendency is called surface tension. Web have you.
Explained !! Why Water Droplets are formed on Surface of cold bottle in
Just think of when it rains and you see tiny droplets of water on the. Web this tendency is called surface tension. In this experiment we can figure out how many drops of water can fit on a. Rinse a penny in tap water. Web the inward pull from the attractions of the molecules results in the smallest possible surface.
The water making a perfect dome. mildlyinteresting
Web when water is dropped carefully onto the surface of a penny, it can pile up into a dome shape before spilling over the small lip. How man) drops of water fit on the penny without flowing over. Web why does water form a dome on a penny? Water’s cohesion and surface tension are special because of hydrogen. Try this.
Web why does water form a dome on top of a penny? Web surface tension is caused by the polar nature of the water molecule. Web you have probably noticed that water can form tiny droplets or beads on many surfaces. Dry thoroughly with a paper towel. This water can stay above the glass because of. Web the inward pull from the attractions of the molecules results in the smallest possible surface for a volume of water, which is a. Web it’s what gives the water the domed look on the penny. Web this cling, or adhesion, of molecules forms the net called surface tension. Water has strong surface tension because of its polarity. Rinse a penny in tap water. Just think of when it rains and you see tiny droplets of water on the. Water molecules are polar, meaning that. Fill a beaker with 25. Water’s cohesion and surface tension are special because of hydrogen. Web have you wondered how many drops of water can fit on a penny? Web why does a penny have so many drops of water? The polar charges are caused by electron. Web once the water has reached the edge of the penny, a dome shape begins to form. Web 4.what property of water allows the water to stick to the penny? This is due to the surface tension.
Web Once The Water Has Reached The Edge Of The Penny, A Dome Shape Begins To Form.
Web this cling, or adhesion, of molecules forms the net called surface tension. The polar charges are caused by electron. This is due to the surface tension. Web you have probably noticed that water can form tiny droplets or beads on many surfaces.
Web Why Does A Penny Have So Many Drops Of Water?
Web here used plain water, soapy & salty water to make a dome on the coin. Web the cohesion and surface tension of water becomes apparent when the drops of water you add to the penny reach the penny’s edge. The cohesion and surface tension of water becomes apparent when the. Water’s cohesion and surface tension are special because of hydrogen.
Web This Video Explores Some Physical Properties Of Water Which Allow A Large Volume Of Water To Dome On The.
Web why does water form a dome on a penny? Web why does water form a dome on top of a penny? Just think of when it rains and you see tiny droplets of water on the. Web assuming there are 20 drops of water in i milliliter, determine:
This Water Can Stay Above The Glass Because Of.
Web the inward pull from the attractions of the molecules results in the smallest possible surface for a volume of water, which is a. Water molecules are polar, meaning that. Web surface tension is caused by the polar nature of the water molecule. Place the penny on a fresh paper towel.