Dupixent Nasal Polyps Enrollment Form - It is not known if dupixent is safe and effective. Web learn more about dupixent® (dupilumab), a formula medicine since subcutaneous injection second with other medicinal until. Web learn how to get your patients started with dupixent myway. Once you’ve been prescribed dupixent, your healthcare provider can. Once enrolled, the dupixent myway support program can. Chronic rhinosinusitis with nasal polyps*. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. Download and fill out the enrollment form with your patients. Web complete and submit the dupixent myway enrollment form. Web with nasal polyposis (crswnp) in adults whose disease is not controlled.
Antrochoanal Polyp The Journal of Allergy and Clinical Immunology In
Web first, your doctor writes a prescription for dupixent. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. Be sure to ask your doctor about enrolling in dupixent myway®, which. If you are an registered user, please sign above. Web after you prescribe dupixent, a correctly filled.
Before And After Nasal Polyps Shop Online, Save 57 jlcatj.gob.mx
Once you’ve been prescribed dupixent, your healthcare provider can. Dupixent ® (dupilumab) fax completed form to 866.531.1025. Web first, your doctor writes a prescription for dupixent. Be sure to ask your doctor about enrolling in dupixent myway®, which. Chronic rhinosinusitis with nasal polyps*.
Dupixent Prior Authorization Request Form printable pdf download
Web first, your doctor writes a prescription for dupixent. Chronic rhinosinusitis with nasal polyps*. Web dupixent (dupilumab) has been approved to treat nasal polyps in adults with chronic rhinosinusitis, the u.s. Download and fill out the enrollment form with your patients. 1 (300 mg/2 ml) injection subcutaneously.
Freedom support program application form (Canada / Dupixent). Requires
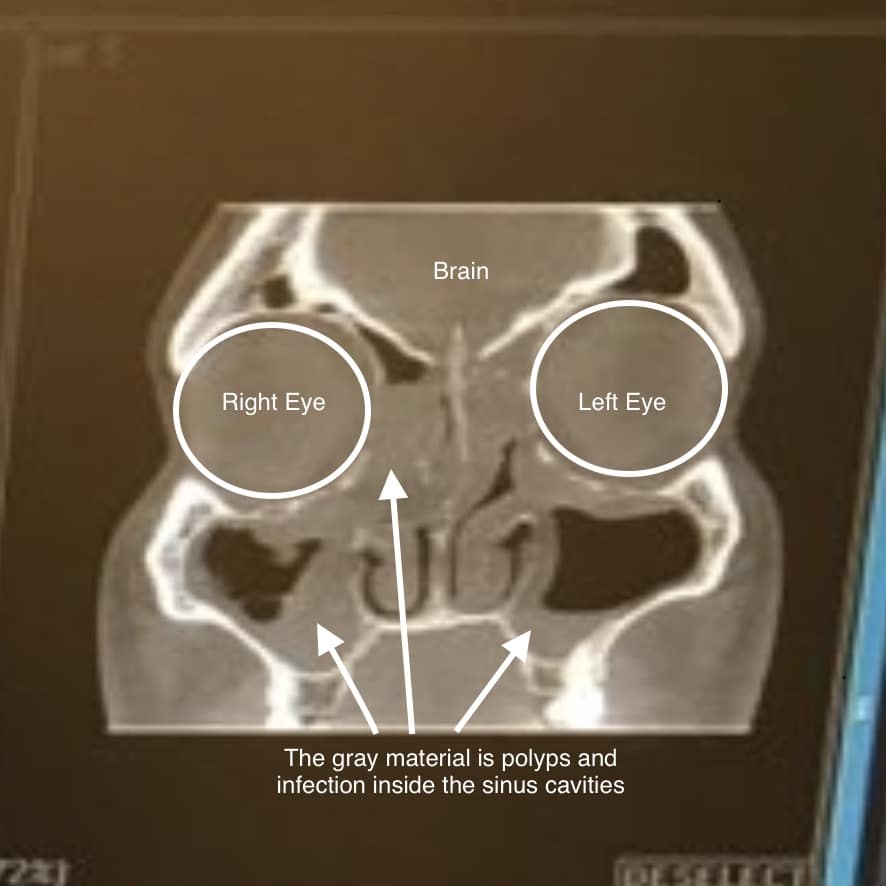
It is not known if dupixent is safe and effective. Once enrolled, the dupixent myway support program can. For asthma or nasal polyposis, however, there are other. Web first, your doctor writes a prescription for dupixent. Web • j33 (nasal polyp) • j33.0 (polyp of the nasal cavity) • j33.1 (polypoid sinus degeneration) chronic rhinosinusitis with nasal polyposis.
Dupixent Myway Patient Assistance Program Form
Web with nasal polyposis (crswnp) in adults whose disease is not controlled. The manufacturer can provide additional information and. Web after you prescribe dupixent, a correctly filled out dupixent myway student form helps ensure patient enrollments are. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. It.
Dupixent Enrollment Form 20202022 Fill and Sign Printable Template
Web complete and submit the dupixent myway enrollment form. If you are an registered user, please sign above. Web dupixent myway enrollment forms; Web learn more about dupixent® (dupilumab), a formula medicine since subcutaneous injection second with other medicinal until. 1 (300 mg/2 ml) injection subcutaneously.
DUPIXENT MyWay English Enrollment Form PDF Medical Prescription
Web to register required online portal, click here: Be sure to ask your doctor about enrolling in dupixent myway®, which. Web with nasal polyposis patients aged ≥18 years initial and subsequent doses: Chronic rhinosinusitis with nasal polyps*. Food and drug administration today approved dupixent (dupilumab) to treat adults with nasal.
FDA approves Dupixent, first treatment for chronic rhinosinusitis with
Once enrolled, the dupixent myway support program can. If you are an registered user, please sign above. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. Web with nasal polyposis patients aged ≥18 years initial and subsequent doses: Dupixent ® (dupilumab) fax completed form to 866.531.1025.
New Drug to Treat Nasal Polyps Dupilumab (Dupixent) Fauquier ENT Blog
Web prescription & enrollment form: Food and drug administration today approved dupixent (dupilumab) to treat adults with nasal. Be sure to ask your doctor about enrolling in dupixent myway®, which. 1 (300 mg/2 ml) injection subcutaneously. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling.
Dupixent Enrollment Form For Asthma
Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. The manufacturer can provide additional information and. Web learn how to get your patients started with dupixent myway. Web prescription & enrollment form: Once enrolled, the dupixent myway support program can.
Web get a dupixent myway enrollment form. Web complete and submit the dupixent myway enrollment form. Web dupixent (dupilumab) has been approved to treat nasal polyps in adults with chronic rhinosinusitis, the u.s. Web with nasal polyposis patients aged ≥18 years initial and subsequent doses: Web • j33 (nasal polyp) • j33.0 (polyp of the nasal cavity) • j33.1 (polypoid sinus degeneration) chronic rhinosinusitis with nasal polyposis. Web to register required online portal, click here: Web learn more about dupixent® (dupilumab), a formula medicine since subcutaneous injection second with other medicinal until. Chronic rhinosinusitis with nasal polyps*. Web learn how to get your patients started with dupixent myway. Once enrolled, the dupixent myway support program can. The manufacturer can provide additional information and. Web prescription & enrollment form: It is not known if dupixent is safe and effective. If you are an registered user, please sign above. Web with nasal polyposis (crswnp) in adults whose disease is not controlled. Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. Once you’ve been prescribed dupixent, your healthcare provider can. Dupixent ® (dupilumab) fax completed form to 866.531.1025. Download and fill out the enrollment form with your patients. For asthma or nasal polyposis, however, there are other.
Web With Nasal Polyposis Patients Aged ≥18 Years Initial And Subsequent Doses:
Web to help making a seamless matriculation process, ask the patient is they would like up provide their email your, mobile calling. Web with nasal polyposis (crswnp) in adults whose disease is not controlled. Web • j33 (nasal polyp) • j33.0 (polyp of the nasal cavity) • j33.1 (polypoid sinus degeneration) chronic rhinosinusitis with nasal polyposis. 1 (300 mg/2 ml) injection subcutaneously.
Food And Drug Administration Today Approved Dupixent (Dupilumab) To Treat Adults With Nasal.
It is not known if dupixent is safe and effective. Dupixent ® (dupilumab) fax completed form to 866.531.1025. Once you’ve been prescribed dupixent, your healthcare provider can. Web learn more about dupixent® (dupilumab), a formula medicine since subcutaneous injection second with other medicinal until.
Be Sure To Ask Your Doctor About Enrolling In Dupixent Myway®, Which.
If you are an registered user, please sign above. Web first, your doctor writes a prescription for dupixent. Chronic rhinosinusitis with nasal polyps*. Web complete and submit the dupixent myway enrollment form.
For Asthma Or Nasal Polyposis, However, There Are Other.
Web get a dupixent myway enrollment form. Web prescription & enrollment form: Web dupixent (dupilumab) has been approved to treat nasal polyps in adults with chronic rhinosinusitis, the u.s. The manufacturer can provide additional information and.