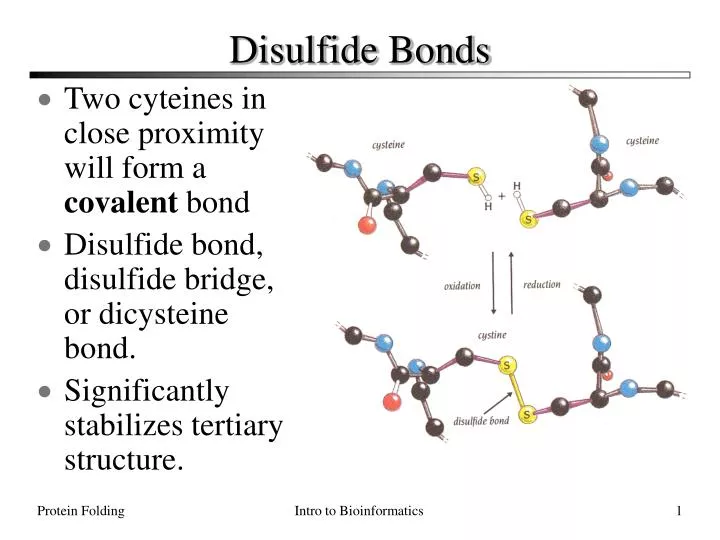
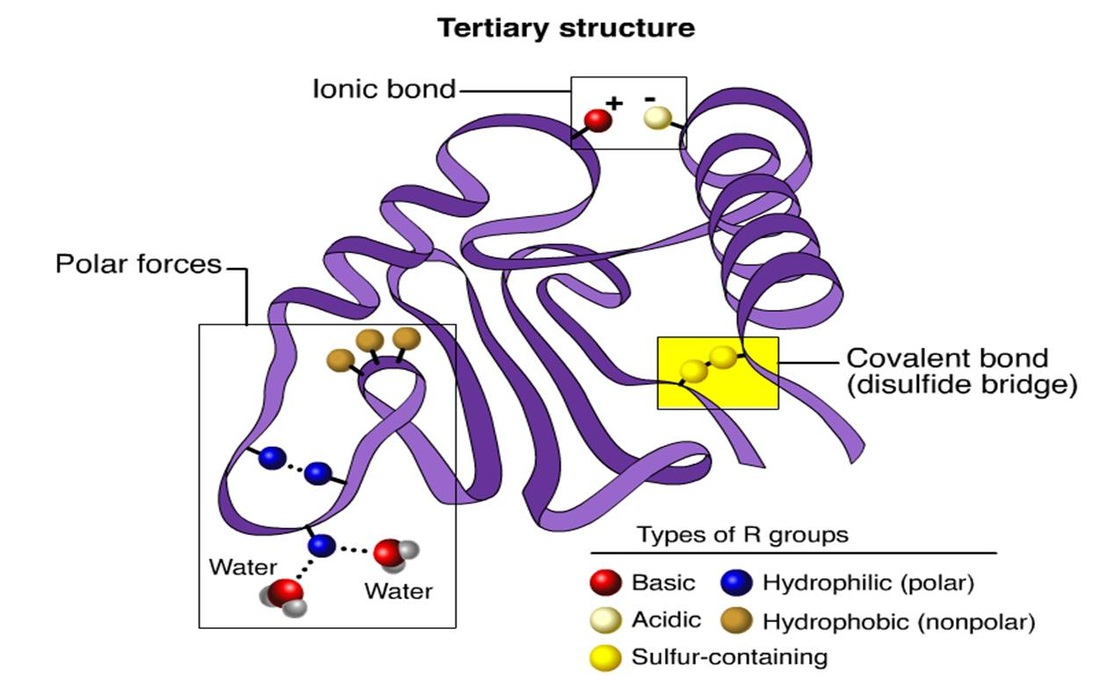
Can Methionine Form Disulfide Bonds - Web disulfide bonds are of two types: Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. Web disulfide bond formation involves a reaction between the sulfhydryl (sh) side chains of two cysteine residues: What bonds are in methionine? Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web is cysteine the only amino acid that can form disulfide bonds? Web oxidation of cysteine (cys) and methionine (met) residues is relatively well understood (reviewed) 19,20, but. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol.
Arrangement of disulfide bonds in mature proteins. Download
Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form.
Disulfide bond wikidoc
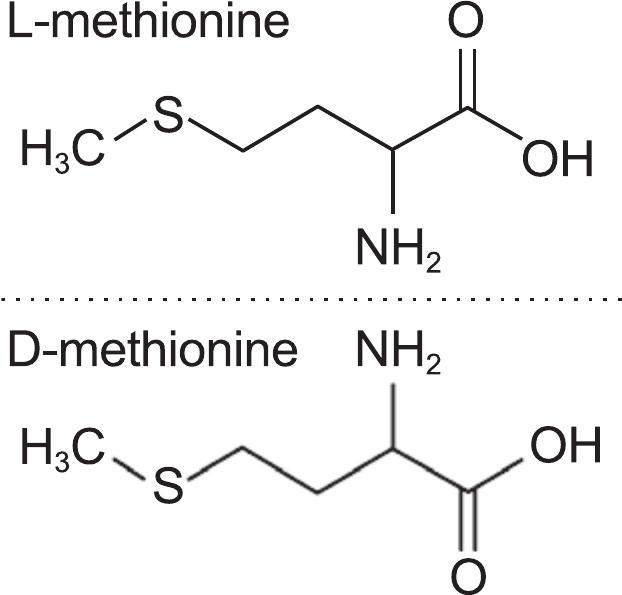
An s − anion from. If you look at the structure of methionine, you won't find s. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. What bonds are in methionine? Web disulfide bridges establish a fundamental element in the molecular architecture of proteins and peptides.
Why Can'T Methionine Form Disulfide Bonds? The 8 Top Answers
Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web answer (1 of 4): Web disulfide bridges establish a fundamental element in the molecular architecture of proteins and.
PPT Disulfide Bonds PowerPoint Presentation ID165240
Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so. Web disulfide bonds are of two types: Web cysteine residues function in the catalytic cycle of.
Potential Mechanisms for Protective Effect of DMethionine on Plasmid
Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. If you look at the structure of methionine, you won't find s. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web disulfide bridges.
Disulfide bond formation protein B Alchetron, the free social
Web disulfide bond formation involves a reaction between the sulfhydryl (sh) side chains of two cysteine residues: Web answer (1 of 4): What bonds are in methionine? Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web oxidation of cysteine (cys) and methionine (met) residues is.
Protein BIOLOGY4ISC
Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can.
Thioldisulfide exchange is involved in the catalytic mechanism of
Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. An s − anion from. Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. Web here, we show that oxidative modification of cysteine side chains by glutathionylation, nitrosylation, and..
The structure of methionine, homocysteine,
Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web disulfide bridges establish a fundamental element in the molecular architecture of proteins and peptides. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so. Web cysteine residues function in.
Along came a spider Digital World Biology
An s − anion from. If you look at the structure of methionine, you won't find s. Web oxidation of cysteine (cys) and methionine (met) residues is relatively well understood (reviewed) 19,20, but. Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. Web answer (1 of 4):
If you look at the structure of methionine, you won't find s. What bonds are in methionine? Web here, we show that oxidative modification of cysteine side chains by glutathionylation, nitrosylation, and. Web is cysteine the only amino acid that can form disulfide bonds? Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so. Web disulfide bonds are of two types: Web oxidation of cysteine (cys) and methionine (met) residues is relatively well understood (reviewed) 19,20, but. Web answer (1 of 4): Web disulfide bridges establish a fundamental element in the molecular architecture of proteins and peptides. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web disulfide bond formation involves a reaction between the sulfhydryl (sh) side chains of two cysteine residues: Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. An s − anion from.
Web Cysteine Residues Function In The Catalytic Cycle Of Many Enzymes, And They Can Form Disulfide Bonds That.
Web here, we show that oxidative modification of cysteine side chains by glutathionylation, nitrosylation, and. What bonds are in methionine? Web disulfide bridges establish a fundamental element in the molecular architecture of proteins and peptides. Web answer (1 of 4):
Web Is Cysteine The Only Amino Acid That Can Form Disulfide Bonds?
Web disulfide bond formation involves a reaction between the sulfhydryl (sh) side chains of two cysteine residues: If you look at the structure of methionine, you won't find s. Web cysteine residues function in the catalytic cycle of many enzymes, and they can form disulfide bonds that. Web the cysteine amino acid group is the only amino acid capable of forming disulfide bonds, and thus can only do so.
Web Oxidation Of Cysteine (Cys) And Methionine (Met) Residues Is Relatively Well Understood (Reviewed) 19,20, But.
Web methionine and cysteine are the only two amino acids within the naturally occurring twenty that contain a sulfur atom in the. An s − anion from. Web disulfide bonds are of two types: Web reply ( 1) thank you sir like (0) > narayan singh best answer disulfide bonds in proteins are formed between the thiol.