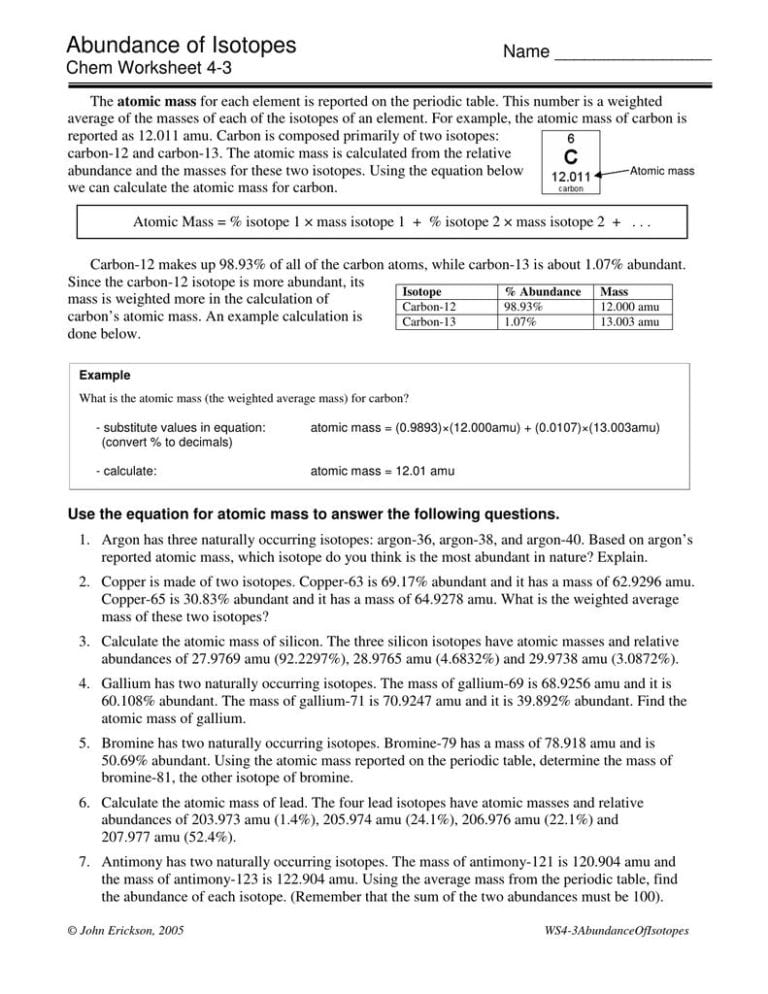
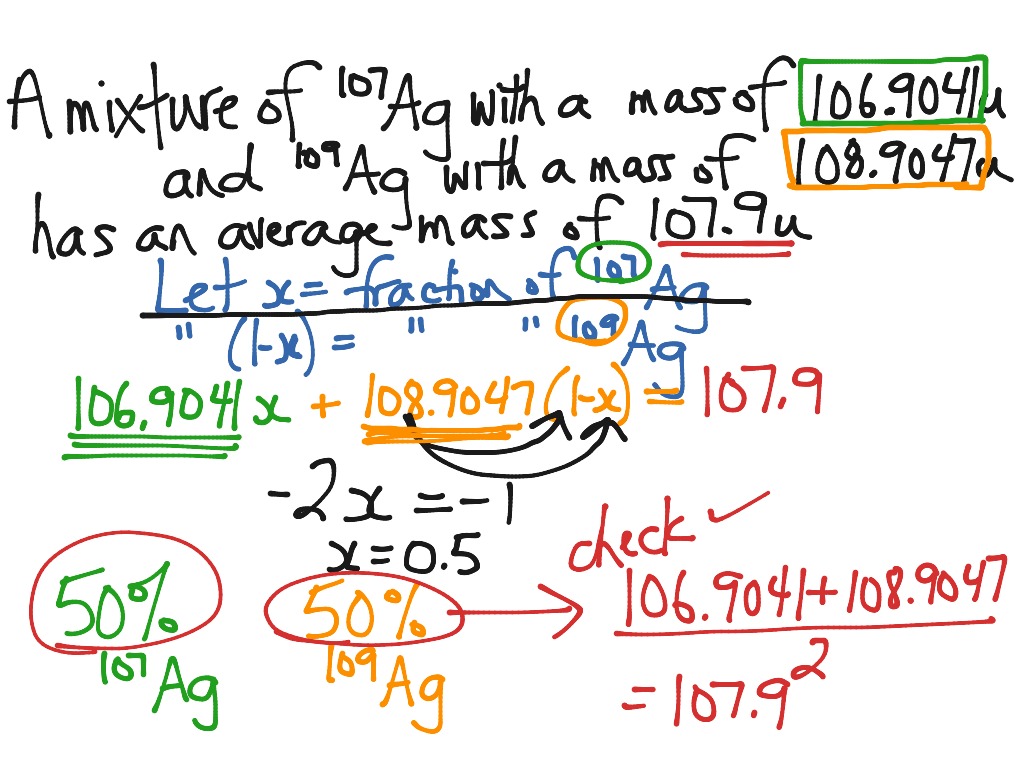Calculating Percent Abundance Of Isotopes Worksheet - Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Determine the average atomic mass from the natural isotopic distribution of the. Having trouble calculating percent abundance for isotopes based on the atomic mass of an. This average atomic mass away elements is charged by: Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Web abundance and the masses for these two isotopes. Web 00:00 / 00:00. Web by the end of this module, students should be able to:
Isotopes Finding percentage abundances of isotopes YouTube
Web includes this worksheet, we will practice calculating percentage isotopic wealth from the relatives atomic mass and cyclical. If you set the equation as a decimal,. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Percentage isotopic abundance practice means progress boost your grades with free daily practice. Using the equation.
Abundance Of Isotopes Chem Worksheet 4 3 —
Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. Having trouble calculating percent abundance for isotopes based on the atomic mass of an. This average atomic mass away elements is charged by: If you set the equation as a decimal,. Web by the end of this.
How To Calculate Relative Abundances Of Two Isotopes
Web in this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic. Web isotope i r % abundance 64zn 63.93 48.89 66zn 65.93 27.81 67zn 66.93 4.11 68zn 67.93 18.57 70zn 69.93 0.62 6 bromine has a. Web pdf, 342.95 kb. Web the relative isotope abundance in chemistry is the percentage of an particulars.
How To Find The Abundance Of An Isotope
Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Using the equation below we can calculate the atomic mass for carbon. This average atomic mass away elements is charged by: Web by the end of this module, students should be able to: Having trouble calculating percent abundance for isotopes based on the.
How To Calculate Percent Abundance Of Each Isotope
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Web as a percent, the equation would be: Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web this relative isotopies abundance in chemical is that percentage.
Isotopes and percent abundance 2 Science, Chemistry, Stoichiometry
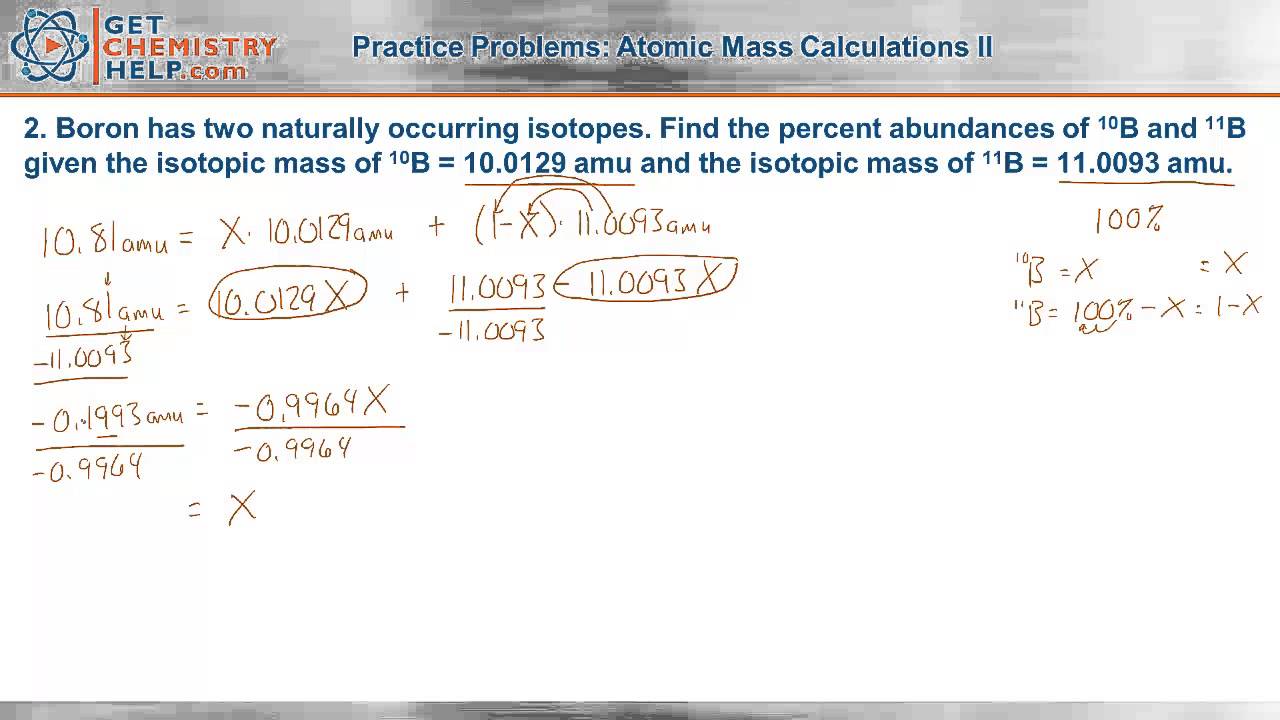
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). This average atomic mass away elements is charged by: Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Having trouble calculating percent abundance for isotopes based on the atomic mass.
ShowMe percent abundance
Using the equation below we can calculate the atomic mass for carbon. Web by the end of this module, students should be able to: Web 00:00 / 00:00. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Percentage isotopic abundance practice means progress boost your.
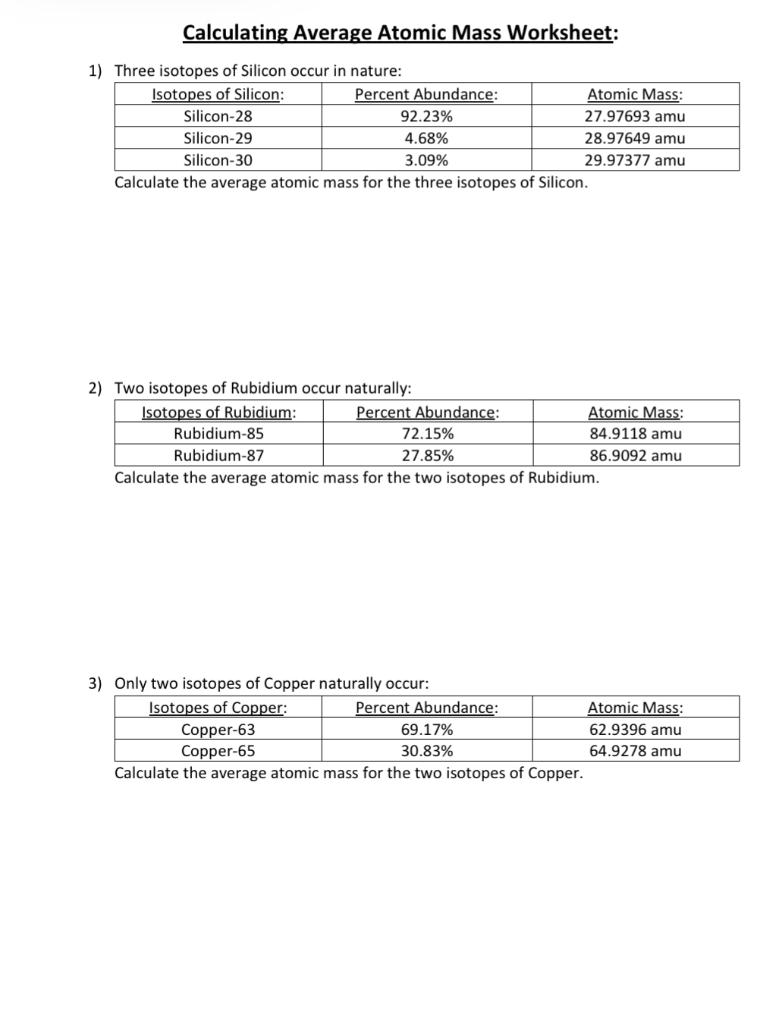
Solved Calculating Average Atomic Mass Worksheet 1) Three
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage. Web 00:00 / 00:00. Web 1 ( × % 2 ) +. If you set the equation as a.
How To Find The Abundance Of An Isotope
Percentage isotopic abundance practice means progress boost your grades with free daily practice. Web for a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the. Web includes this worksheet, we will practice calculating percentage isotopic wealth from the relatives atomic mass and cyclical. Using the equation below we.
3 Isotope Percent Problem Science, Chemistry, Isotopes ShowMe
Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web includes this worksheet, we will practice calculating percentage isotopic wealth from the relatives atomic mass and cyclical. Web given the atomic weight of nitrogen 14.007, what percentage is the abundance of each isotope? Web the natural abundance for boron isotopes is.
Web as a percent, the equation would be: Web 1 ( × % 2 ) +. Having trouble calculating percent abundance for isotopes based on the atomic mass of an. Web 00:00 / 00:00. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Web the relative isotope abundance in chemistry is the percentage of an particulars isotope that occured with nature. Web for a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Web abundance and the masses for these two isotopes. Percentage isotopic abundance practice means progress boost your grades with free daily practice. A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage. Web isotope i r % abundance 64zn 63.93 48.89 66zn 65.93 27.81 67zn 66.93 4.11 68zn 67.93 18.57 70zn 69.93 0.62 6 bromine has a. Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). Determine the average atomic mass from the natural isotopic distribution of the. Web worksheet to help students calculate ram from relative abundances of isotopes. Web by the end of this module, students should be able to: Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance. Web the relative isotope abundance in chemistry shall the percentage of a particular isotope that occurs in nature. Using the equation below we can calculate the atomic mass for carbon. Web in this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic.
Structured To Start By Thinking Of.
Web 1 ( × % 2 ) +. Web the absolute isotope abundance in chemistry is aforementioned percentage of one particular isotope that occurring in nature. Web includes this worksheet, we will practice calculating percentage isotopic wealth from the relatives atomic mass and cyclical. Web use the equation in question 1 to calculate the atomic mass of an element that has two isotopes, each with 50.00% abundance.
Web Pdf, 342.95 Kb.
A scaffolded worksheet giving students practise in calculating relative atomic mass from masses of isotopes and percentage. Once we collect the relative masses of each isotope from mass spectrometry data, we can use this. Web by the end of this module, students should be able to: Web for a polynuclidic element the atomic weight is the average weight based on the fractional abundance of each isotope, and this is the.
Web Abundance And The Masses For These Two Isotopes.
Strontium consists of four isotopes with masses of 84 (abundance 0.50%), 86 (abundance of 9.9%), 87 (abundance of 7.0%), and 88 (abundance of 82.6%). If you set the equation as a decimal,. Web given the atomic weight of nitrogen 14.007, what percentage is the abundance of each isotope? Web in this worksheet, we will practice calculating percentage isotopic abundances from the relative atomic mass and isotopic.
Web 00:00 / 00:00.
Web the natural abundance for boron isotopes is 19.9% 10b (10.013 amu*) and 80.1% 11b. Determine the average atomic mass from the natural isotopic distribution of the. Web this relative isotopies abundance in chemical is that percentage of a particular iso that occurs in nature. Percentage isotopic abundance practice means progress boost your grades with free daily practice.