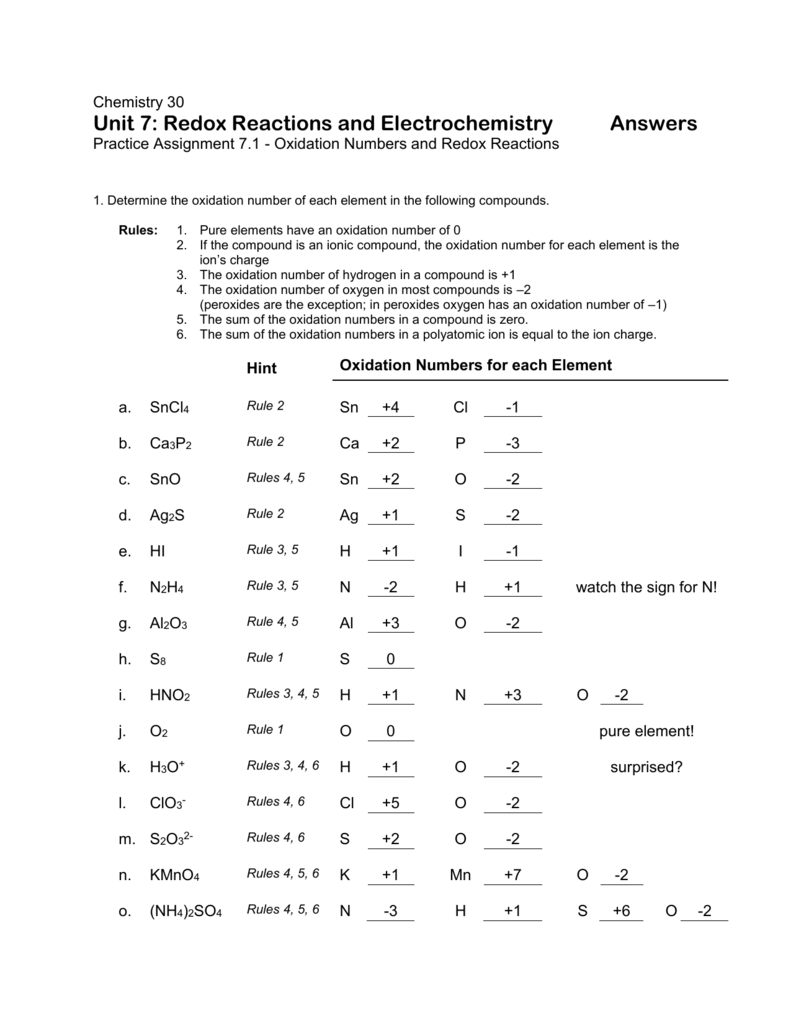
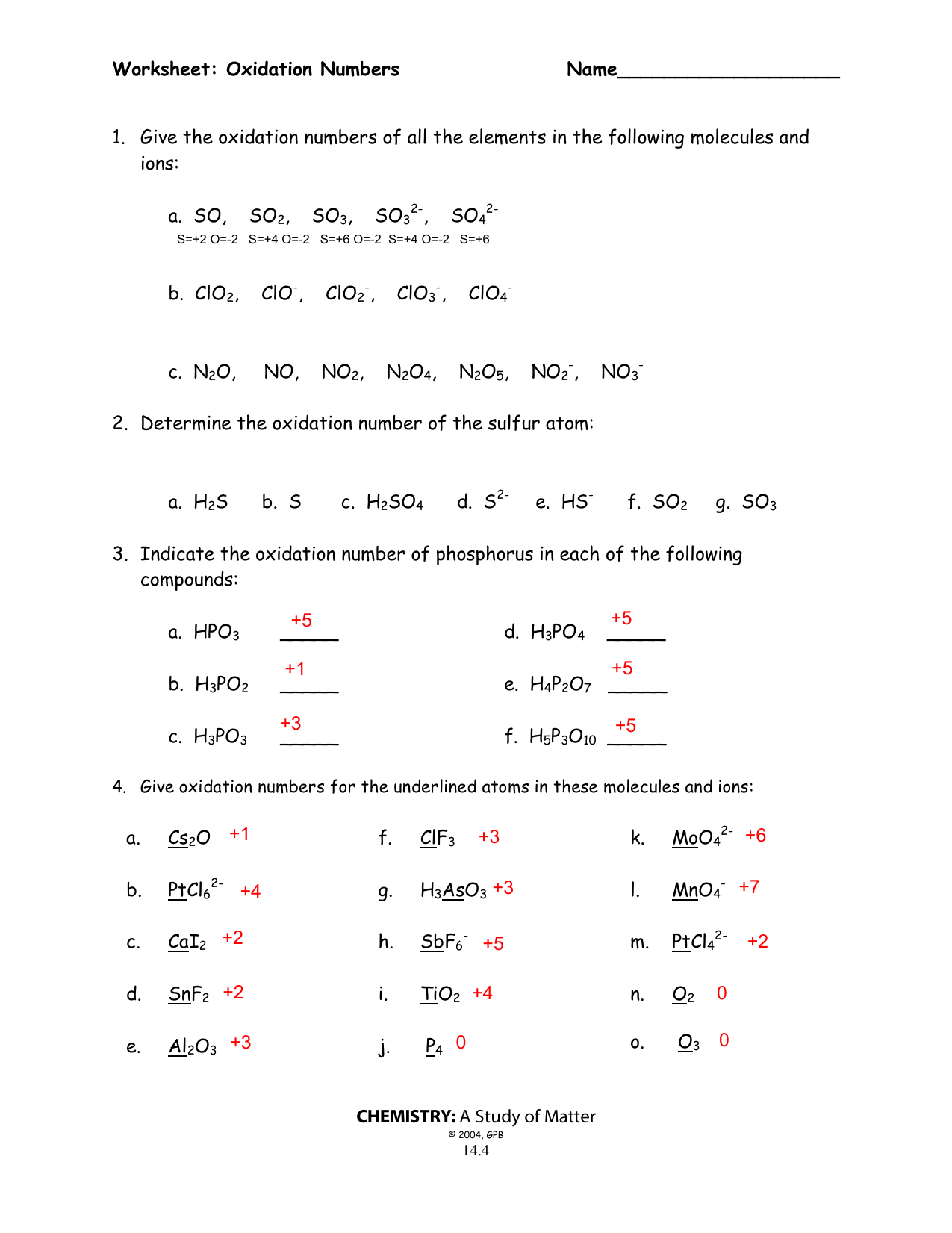
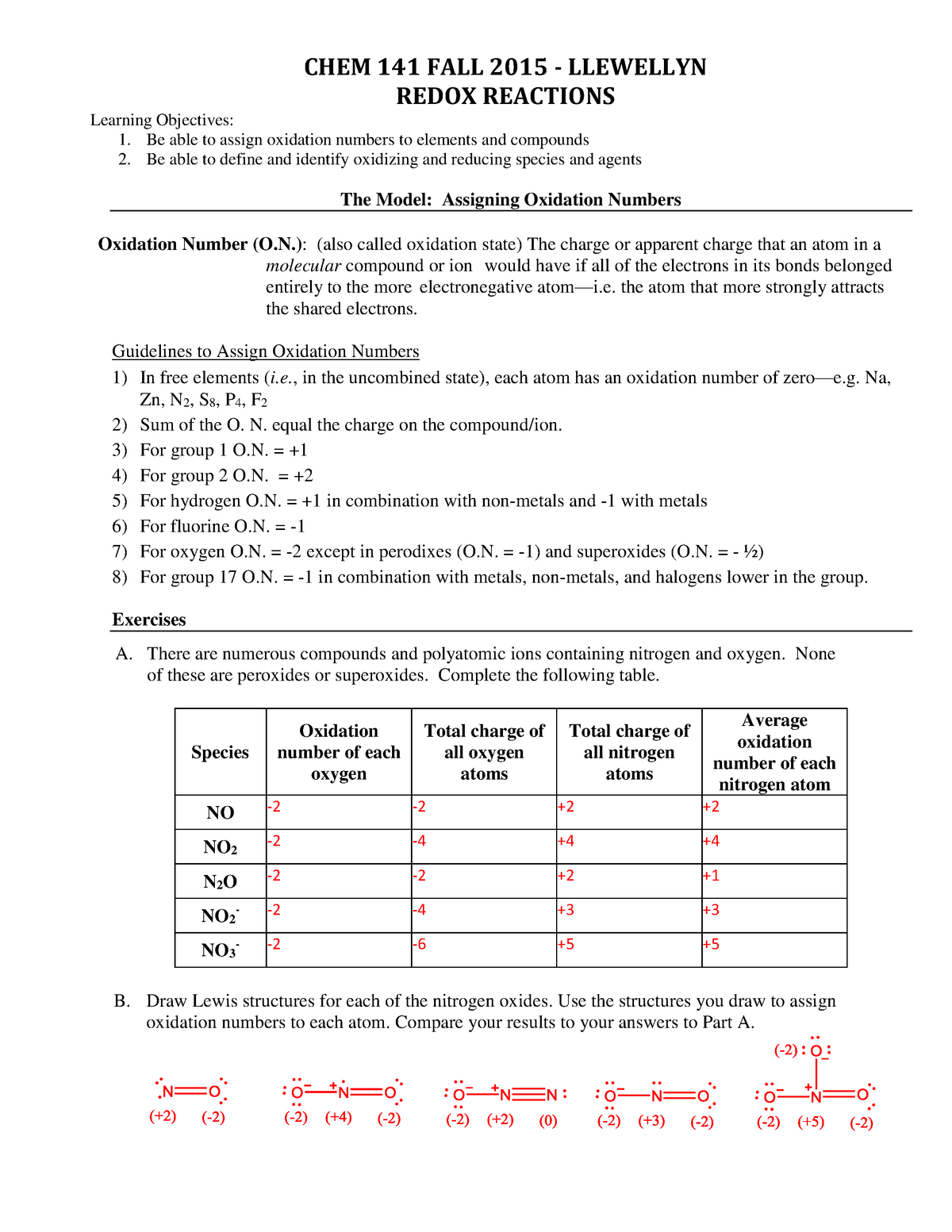
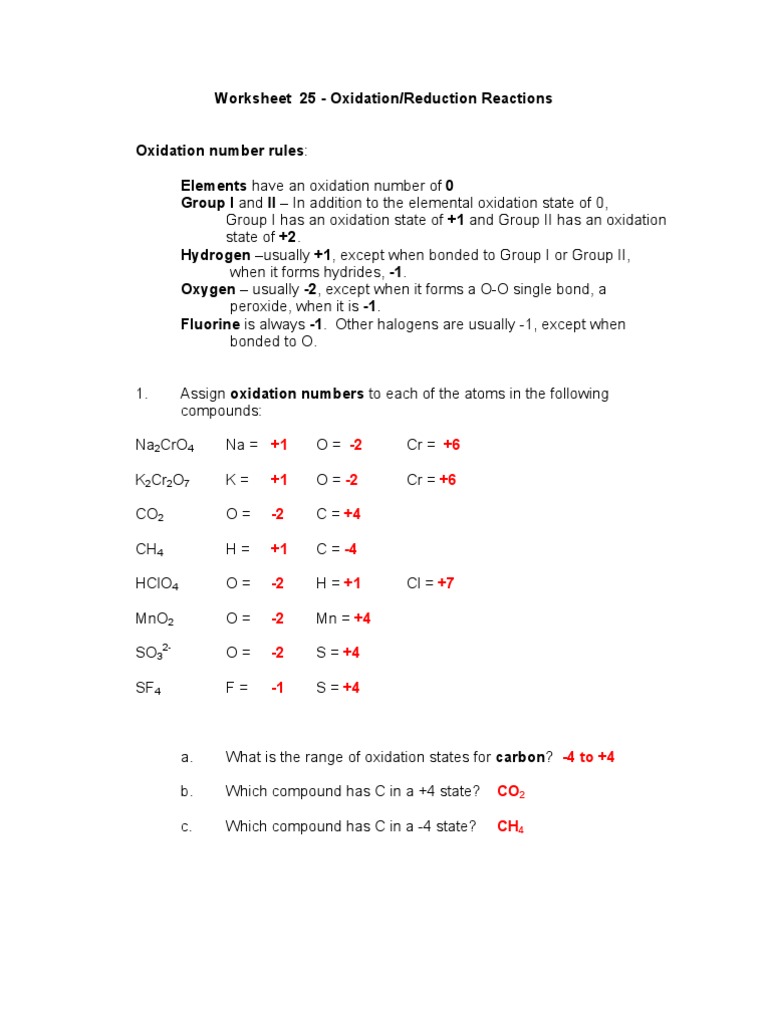
Worksheet Oxidation Numbers Answer Key - Given the following redox reaction: 2 for a monatomic ion, the oxidation number is the charge on the ion. In the following questions, give the oxidation number of the indicated atoms/ion. Web oxidation numbers worksheet worksheet. The handout outlines and illustrates the rules for assigning oxidation numbers. Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Give the oxidation numbers of all the elements in the following. Web this product contains two pages. Web the worksheet contains 20 examples that cover all of the oxidation number rules discussed in class. In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby.
Worksheet Oxidation Numbers Answer Key Ivuyteq
Give the oxidation numbers of all the elements in the following molecules and ions: The oxidation number of an element in a. For atoms in a neutral atom, molecule, etc., the sum of all. A pure element has an oxidation number of 0. Live worksheets > english > chemistry > redox > oxidation numbers worksheet.
Worksheet Oxidation Numbers Answer Key
Web chemists have developed rules used to assign oxidation numbers. The oxidation number is the charge that a central metal atom will have even after all the ligands have been. Web oxidation numbers worksheet worksheet. The handout outlines and illustrates the rules for assigning oxidation numbers. A reaction in which one species transfers electrons to another is called an oxidation.
Oxidation Numbers Practice Worksheet Answers kidsworksheetfun
Live worksheets > english > chemistry > redox > oxidation numbers worksheet. Web this product contains two pages. Give the oxidation numbers of all the elements in the following. N in n 2 o 3 2. A reaction in which one species transfers electrons to another is called an oxidation.
Assigning Oxidation Numbers Worksheet Fill Out and Sign Printable PDF
Web give oxidation numbers for the underlined atoms in these molecules and ions: Web fe d) which species is the being reduced? The handout outlines and illustrates the rules for assigning oxidation numbers. Web chemistry worksheet oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers.
Assigning Oxidation Numbers Worksheet Answer Key Escolagersonalvesgui
S in h 2 so 4 3. Web chemists have developed rules used to assign oxidation numbers. Web fe d) which species is the being reduced? The handout outlines and illustrates the rules for assigning oxidation numbers. Live worksheets > english > chemistry > redox > oxidation numbers worksheet.
Oxidation State Worksheets
Identify the oxidation state of nitrogen in the following: In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby. 2 for a monatomic ion, the oxidation number is the charge on the ion. The handout outlines and illustrates the rules for assigning oxidation numbers. A pure element has an oxidation number of 0.
41 Oxidation Number Worksheet Answer Key Worksheet Master
Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. Assign oxidation numbersto each of the atoms in the following compounds: Live worksheets > english > chemistry > redox > oxidation numbers worksheet. Web redox and oxidation numbers. Oxidation numbers hey name 1.
Answer Key Oxidation Numbers Worksheet Answers Worksheet Resume Examples
Web 2fe(s) + 3cl2(g) → 2fe3 + + 6cl −. The oxidation number of an element in a. The following rules will help you determine the oxidation. For atoms in a neutral atom, molecule, etc., the sum of all. Web the worksheet contains 20 examples that cover all of the oxidation number rules discussed in class.
Worksheet Oxidation Numbers Answer Key Promotiontablecovers
Web chemists have developed rules used to assign oxidation numbers. Name _____ period _____ oxidation number rules: Identify the oxidation state of nitrogen in the following: Web fe d) which species is the being reduced? S in h 2 so 4 3.
Oxidation Numbers Worksheet Answer Key worksheet
Assign oxidation numbersto each of the atoms in the following compounds: Live worksheets > english > chemistry > redox > oxidation numbers worksheet. Web chemists have developed rules used to assign oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers.
The following rules will help you determine the oxidation. Web chemistry worksheet oxidation numbers. Web give oxidation numbers for the underlined atoms in these molecules and ions: For atoms in a neutral atom, molecule, etc., the sum of all. Give the oxidation numbers of all the elements in the following molecules and ions: A reaction in which one species transfers electrons to another is called an oxidation. The oxidation number of an element in a. In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby. 2 for a monatomic ion, the oxidation number is the charge on the ion. The oxidation number is the charge that a central metal atom will have even after all the ligands have been. The state of elements and atoms can be denoted in many ways. Web this product contains two pages. Web 1 the oxidation number of an element is zero. Identify the oxidation state of nitrogen in the following: Web chemists have developed rules used to assign oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers. The handout outlines and illustrates the rules for assigning oxidation numbers. A pure element has an oxidation number of 0. N in n 2 o 3 2. Web this product contains two pages.
Assign Oxidation Numbersto Each Of The Atoms In The Following Compounds:
The oxidation number is the charge that a central metal atom will have even after all the ligands have been. Web chemistry worksheet oxidation numbers. Given the following redox reaction: Web this product contains two pages.
Name _____ Period _____ Oxidation Number Rules:
Give the oxidation numbers of all the elements in the following molecules and ions: S in h 2 so 4 3. In the following questions, give the oxidation number of the indicated atoms/ion. The handout outlines and illustrates the rules for assigning oxidation numbers.
Web Redox And Oxidation Numbers.
In essence, the fe pushes electrons and the cl 2 pulls electrons, thereby. Name _____ period _____ oxidation number rules: The state of elements and atoms can be denoted in many ways. Give the oxidation numbers of all the elements in the following.
A Reaction In Which One Species Transfers Electrons To Another Is Called An Oxidation.
Web give oxidation numbers for the underlined atoms in these molecules and ions: Web fe d) which species is the being reduced? The handout outlines and illustrates the rules for assigning oxidation numbers. The following rules will help you determine the oxidation.