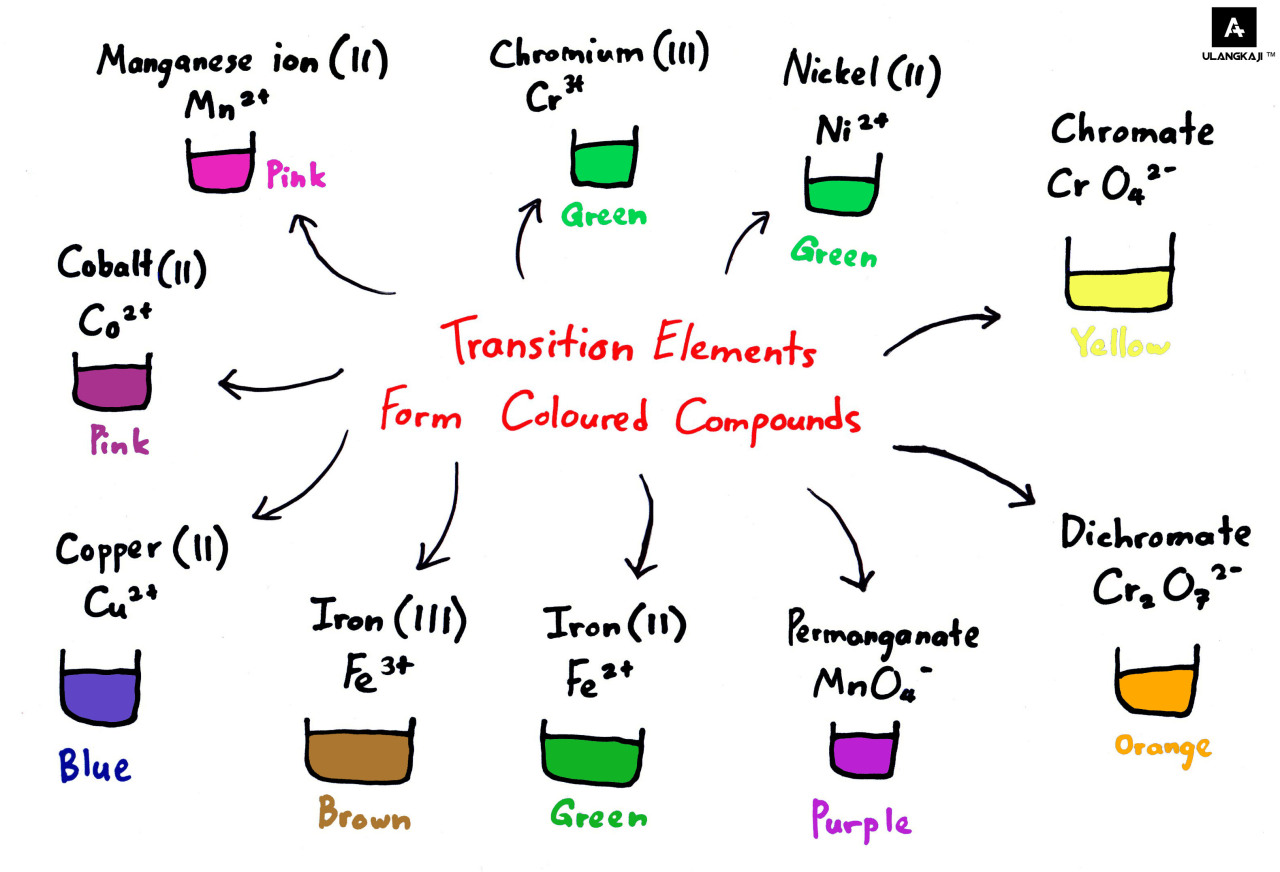
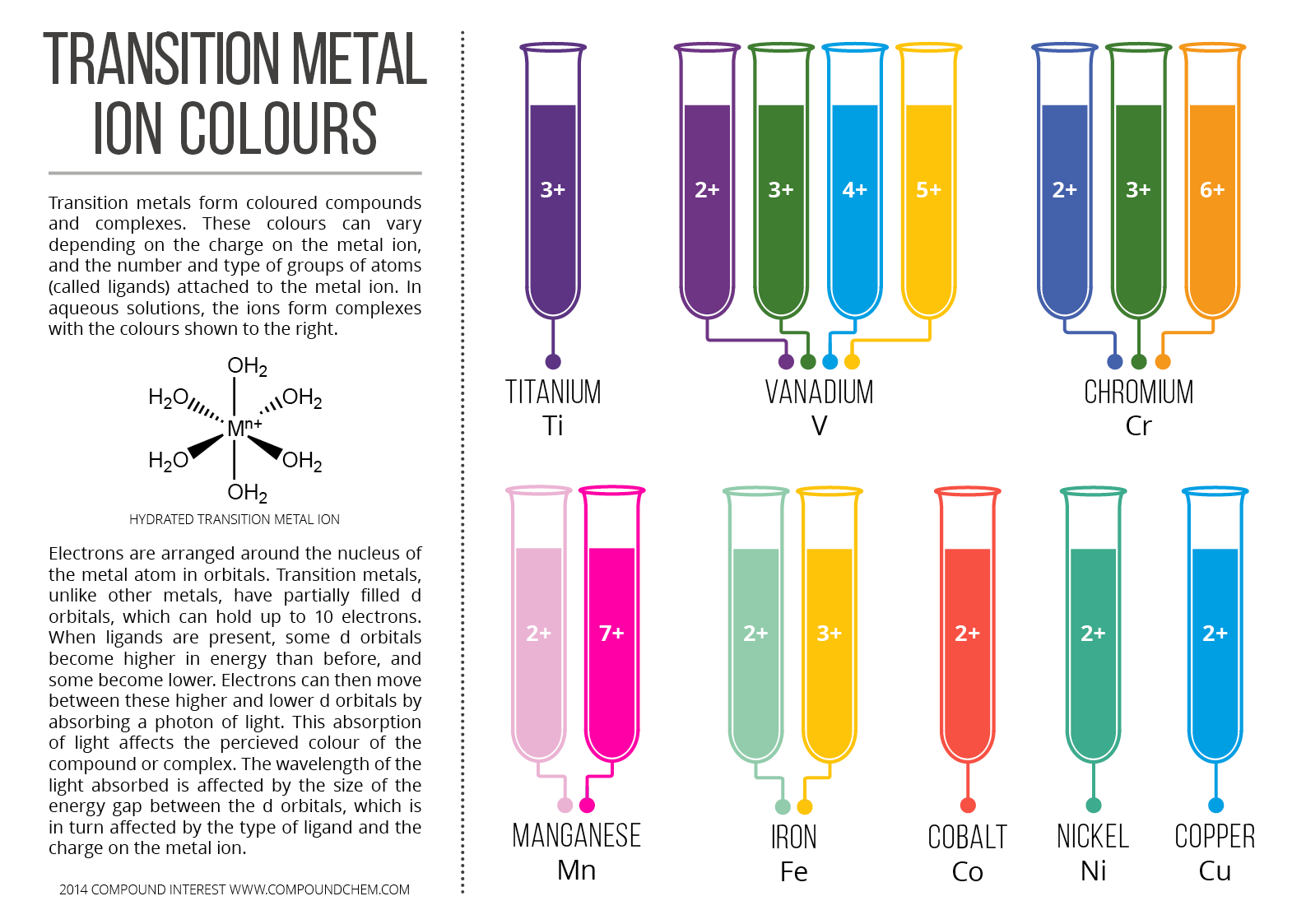
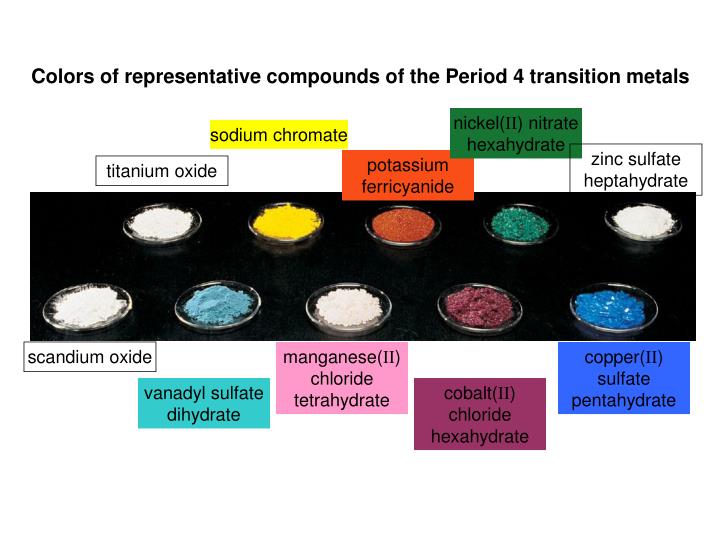
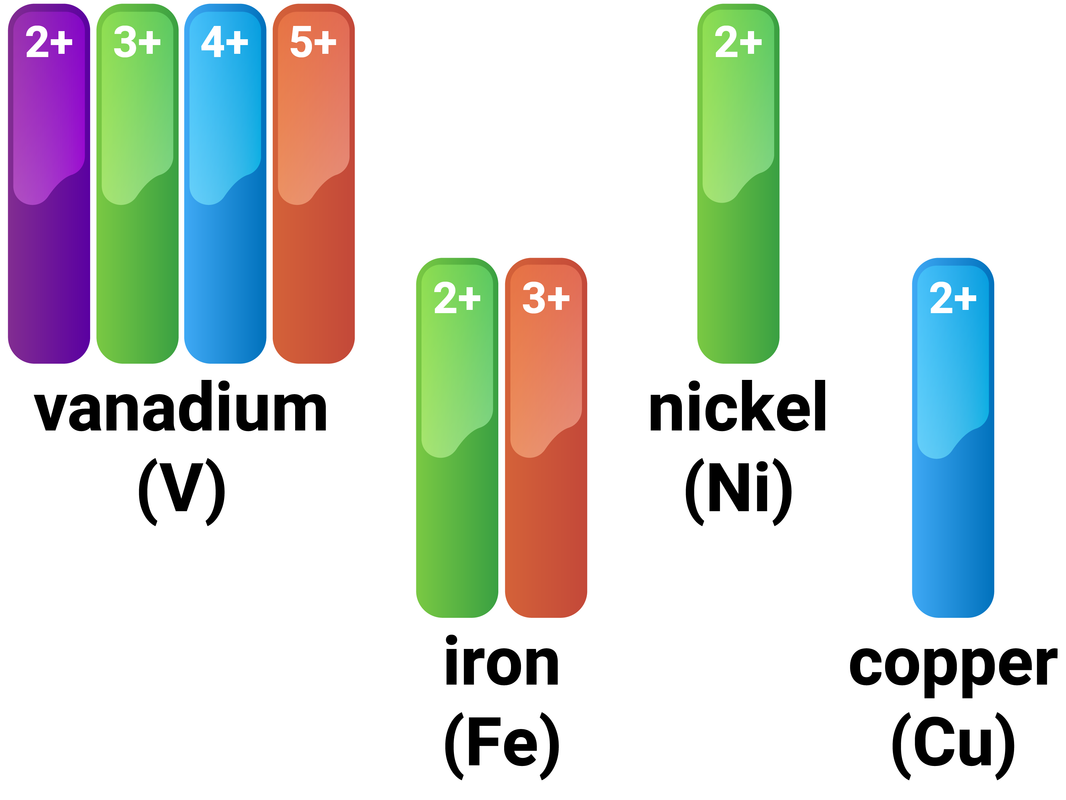
Why Transition Metals Form Coloured Compounds - Web reason for the color of transition metals: The electrons can absorb certain. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Transition metals and their many compounds act as good. Web transition metals can form colored compounds when bonded to other elements due to the energy levels of the d block where electrons can be. Web openstax learning objectives outline the general approach for the isolation of transition metals from natural sources. Web form compounds which are often paramagnetic; Web transition elements have 3d orbitals with the same energy level however when molecules/ligands form dative covalent. Transition metal ions are not coloured on their own. Web the transition metals form coloured compounds due to the promotion of unpaired electrons by visible light.
PPT KS4 Chemistry PowerPoint Presentation, free download ID6307470
The transition metals have five distinct. Web the transition elements are metals. Web no views 1 minute ago. Transition metals and their many compounds act as good. Transition metals, on the other hand, are unique in that the energy difference.
SPMStraightA — Transition Metals make coloured compounds
Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Web this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. Web transition metals the transition metals are in the block in the middle of the periodic table, between.
science chemistry compound transition metals Fundamental Photographs
Web no views 1 minute ago. Web form compounds which are often paramagnetic; Web reason for the color of transition metals: The transition metals have five distinct. Web openstax learning objectives outline the general approach for the isolation of transition metals from natural sources.
PPT Atoms and ElementsThe Nature of Matter PowerPoint Presentation
Web this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. Web transition metals can form colored compounds when bonded to other elements due to the energy levels of the d block where electrons can be. Transition metals, on the other hand, are unique in.
PPT Properties of Transition Metals PowerPoint Presentation, free
Web the transition metals form coloured compounds due to the promotion of unpaired electrons by visible light. Web transition elements have 3d orbitals with the same energy level however when molecules/ligands form dative covalent. Web openstax learning objectives outline the general approach for the isolation of transition metals from natural sources. The colour of the compound depends on the energy.
PPT Transition Metals and Color PowerPoint Presentation, free
Web transition metals can form colored compounds when bonded to other elements due to the energy levels of the d block where electrons can be. Web reason for the color of transition metals: Web explain why transition metals form coloured compounds when bonded to a ligand. Web openstax learning objectives outline the general approach for the isolation of transition metals.
Why compounds of transition metals are coloured? Socratic
Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Outlining why and how transition metal ions form coloured compounds. Transition metal ions are not coloured on their own. The electrons can absorb certain.
PPT The Period 4 transition metals PowerPoint Presentation ID3038336
Web transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. Web this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. Web the transition metals generally form coloured compounds. Web the transition metals.
PPT Transition Metal Chemistry PowerPoint Presentation, free download
Web this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. Web explain why transition metals form coloured compounds when bonded to a ligand. The transition metals have five distinct. Transition metal ions are not coloured on their own. Transition metals and their many compounds.
Identifying the products of chemical reactions (chemistry only) OCR
The colour of the compound depends on the energy of. Web no views 1 minute ago. It is only when they form complexes with other ions or molecules that they. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Web transition metals have high melting points and densities, form coloured compounds and act.
Web transition elements have 3d orbitals with the same energy level however when molecules/ligands form dative covalent. The transition metals have five distinct. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Web form compounds which are often paramagnetic; Web the transition elements are metals. The electrons can absorb certain. Web transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. Transition metal ions are not coloured on their own. Web openstax learning objectives outline the general approach for the isolation of transition metals from natural sources. Web no views 1 minute ago. Web the transition metals form coloured compounds due to the promotion of unpaired electrons by visible light. Web transition metals can form colored compounds when bonded to other elements due to the energy levels of the d block where electrons can be. They have high melting points and densities, and are strong and hard. Web explain why transition metals form coloured compounds when bonded to a ligand. It is only when they form complexes with other ions or molecules that they. Web this page explains what a transition metal is in terms of its electronic structure, and then goes on to look at the general features of. Web the transition metals generally form coloured compounds. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts. Transition metals, on the other hand, are unique in that the energy difference. Web transition metals have high melting points and densities, form coloured compounds and act as catalysts.
Web Transition Metals Have High Melting Points And Densities, Form Coloured Compounds And Act As Catalysts.
The colour of the compound depends on the energy of. Web the transition metals form coloured compounds due to the promotion of unpaired electrons by visible light. They have high melting points and densities, and are strong and hard. The transition metals have five distinct.
Web Transition Metals Have High Melting Points And Densities, Form Coloured Compounds And Act As Catalysts.
Web reason for the color of transition metals: Web transition metals the transition metals are in the block in the middle of the periodic table, between groups 2 and 3. Transition metals, on the other hand, are unique in that the energy difference. Web openstax learning objectives outline the general approach for the isolation of transition metals from natural sources.
Web No Views 1 Minute Ago.
Web transition metals can form colored compounds when bonded to other elements due to the energy levels of the d block where electrons can be. Transition metal ions are not coloured on their own. It is only when they form complexes with other ions or molecules that they. Transition metals and their many compounds act as good.
Web The Transition Elements Are Metals.
Web form compounds which are often paramagnetic; Web transition elements have 3d orbitals with the same energy level however when molecules/ligands form dative covalent. Outlining why and how transition metal ions form coloured compounds. The electrons can absorb certain.