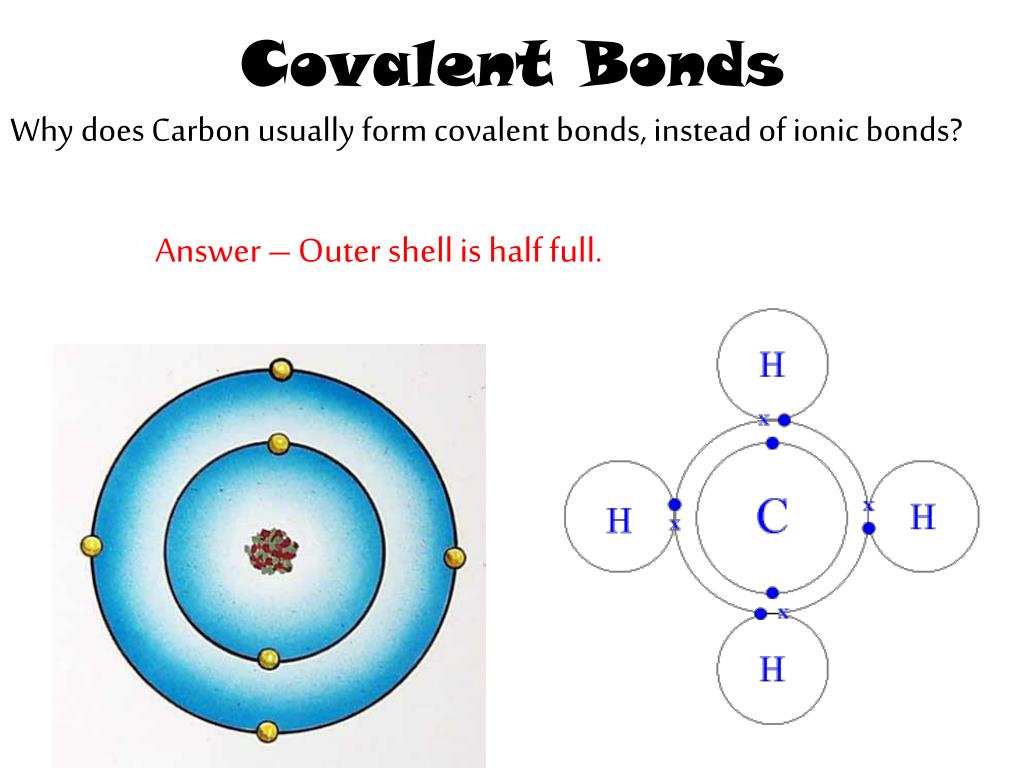
Why Does Carbon Form Covalent Bonds - Web carbon forms covalent bonds with atoms of carbon or other elements. Web carbon atoms in diamond form a 'tetrahedral' arrangement properties and uses. Web why does carbon form covalent bonds? Web the most common type of bond formed by carbon is a covalent bond. Web explain properties of carbon. Web why form chemical bonds? Carbon atoms can join together to make molecules with very long chains of. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web four covalent bonds can be formed by carbon. The electrons involved are in the outer shells.
PPT Biochemistry PowerPoint Presentation, free download ID89333
In most cases, carbon shares electrons with other atoms. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Web the most common type of bond formed by carbon is a covalent bond. Web well, carbon can form up to four covalent bonds. Carbon has both physical and chemical properties to form compounds by.
Carbon to Carbon Single, Double & Triple Bonds Surfguppy
In most cases, carbon shares electrons with other atoms. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). It has an electronic configuration 1 s 2 2 s 2 2 p 2. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many.
PPT Biochemistry PowerPoint Presentation, free download ID89333
Solution carbon carbon has the unique property of catenation (forming a long. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with. Web the most common type of bond formed by carbon is a covalent bond. Web carbon generally forms compounds by covalent bonds because: Therefore, carbon completes its octet.
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
Web so, in order to completely fill the octet carbon atom will combine with atoms that can provide 4 additional electrons to a carbon. Web carbon generally forms compounds by covalent bonds because: For any atom, stability is achieved by following the. With hydrogen, nitrogen, oxygen, and other heteroatoms. Web why does carbon form covalent bonds?
Science class 10 Why carbon form covalent bonds YouTube
Due to the number of electrons present in the last shell, carbon forms covalent compounds. The electrons involved are in the outer shells. Web why does carbon form covalent bonds? Two atoms share a pair of. Web so, in order to completely fill the octet carbon atom will combine with atoms that can provide 4 additional electrons to a carbon.
Four covalent bonds. Carbon has four valence electrons and here a
Web carbon atoms in diamond form a 'tetrahedral' arrangement properties and uses. Two atoms share a pair of. Chemical bonds between nonmetals are known as covalent bonds. Web the most common type of bond formed by carbon is a covalent bond. Solution carbon carbon has the unique property of catenation (forming a long.
hillis2e_ch02
Web why does carbon form covalent bonds? Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). Solution carbon carbon has the unique property of catenation (forming a long. Due to the number of electrons present in the last shell, carbon forms covalent compounds. Chemical bonds between nonmetals are known as covalent bonds.
[Best Answer] How many covalent bonds does carbon form if each of its
Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia). The unique ability of carbon to form bonds with other atoms. For any atom, stability is achieved by following the. Due to the number of electrons present in the last shell, carbon forms covalent compounds. Solution carbon carbon has the unique property of catenation.
PPT 2.1 Nature of Matter PowerPoint Presentation, free download ID
There is a great diversity of carbon compounds, ranging in. Web however, this requires lots of energy and would make the system unstable. Web carbon atoms in diamond form a 'tetrahedral' arrangement properties and uses. Therefore, carbon completes its octet by. The electrons involved are in the outer shells.
Multiple Bonds — Double & Triple Bonds Expii
The unique ability of carbon to form bonds with other atoms. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with. Web the most common type of bond formed by carbon is a covalent bond. Web so, in order to completely fill the octet carbon atom will combine with atoms.
Web carbon atoms in diamond form a 'tetrahedral' arrangement properties and uses. Web carbon forms covalent bonds with atoms of carbon or other elements. Web four covalent bonds can be formed by carbon. In most cases, carbon shares electrons with other atoms. Web why form chemical bonds? Oxygen and other atoms in group 16 obtain. Due to the number of electrons present in the last shell, carbon forms covalent compounds. Web carbon generally forms compounds by covalent bonds because: Web why does carbon form covalent bonds? Web the most common type of bond formed by carbon is a covalent bond. Web explain properties of carbon. With hydrogen, nitrogen, oxygen, and other heteroatoms. Two atoms share a pair of. Web last updated molecular shape isomerism in organic compounds there are many types of chemical bonds and forces. For any atom, stability is achieved by following the. Web two reasons for large number of carbon compounds: Web carbon has an unusual ability to bond to itself. Carbon atoms can join together to make molecules with very long chains of. Carbon has both physical and chemical properties to form compounds by the formation of covalent. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
Two Atoms Share A Pair Of.
Web however, this requires lots of energy and would make the system unstable. The electrons involved are in the outer shells. Therefore, carbon completes its octet by. It has an electronic configuration 1 s 2 2 s 2 2 p 2.
Web Two Reasons For Large Number Of Carbon Compounds:
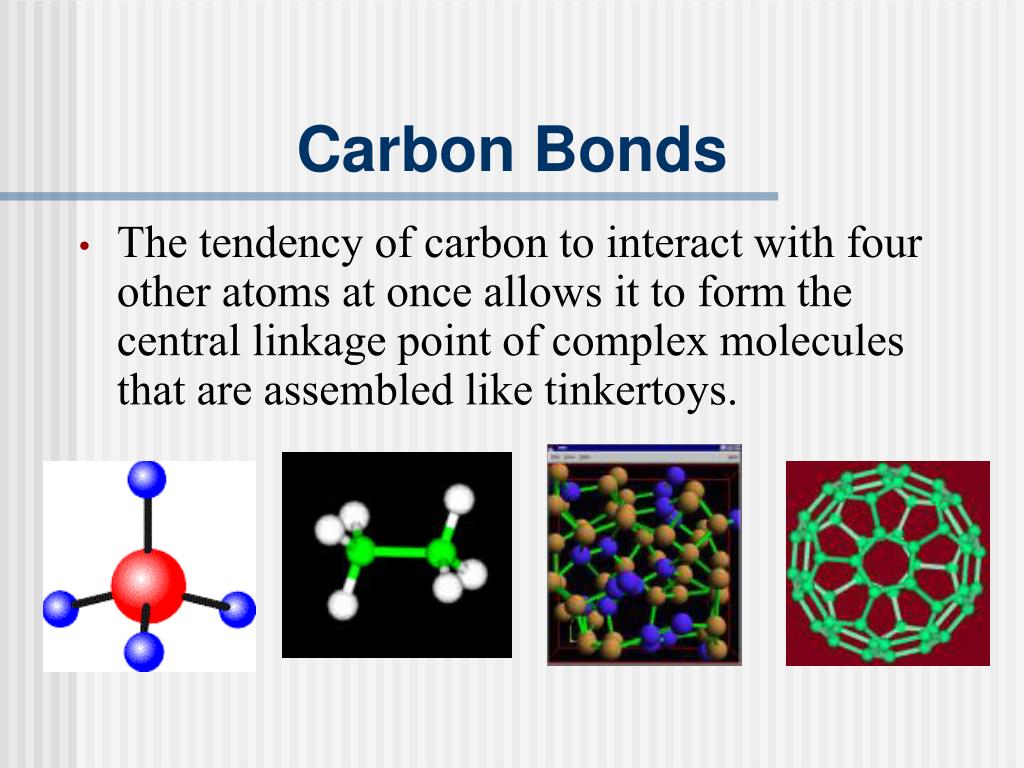
Carbon has both physical and chemical properties to form compounds by the formation of covalent. Oxygen and other atoms in group 16 obtain. Web the four covalent bonding positions of the carbon atom can give rise to a wide diversity of compounds with many functions,. Web to obtain an octet, these atoms form three covalent bonds, as in nh 3 (ammonia).
The Carbon Atom Is Unique Among Elements In Its Tendency To Form Extensive Networks Of Covalent Bonds Not Only With.
There is a great diversity of carbon compounds, ranging in. Web the most common type of bond formed by carbon is a covalent bond. Web carbon forms covalent bonds with atoms of carbon or other elements. Carbon atoms can join together to make molecules with very long chains of.
Web Four Covalent Bonds Can Be Formed By Carbon.
Web carbon atoms in diamond form a 'tetrahedral' arrangement properties and uses. Web why form chemical bonds? Web so, in order to completely fill the octet carbon atom will combine with atoms that can provide 4 additional electrons to a carbon. With hydrogen, nitrogen, oxygen, and other heteroatoms.






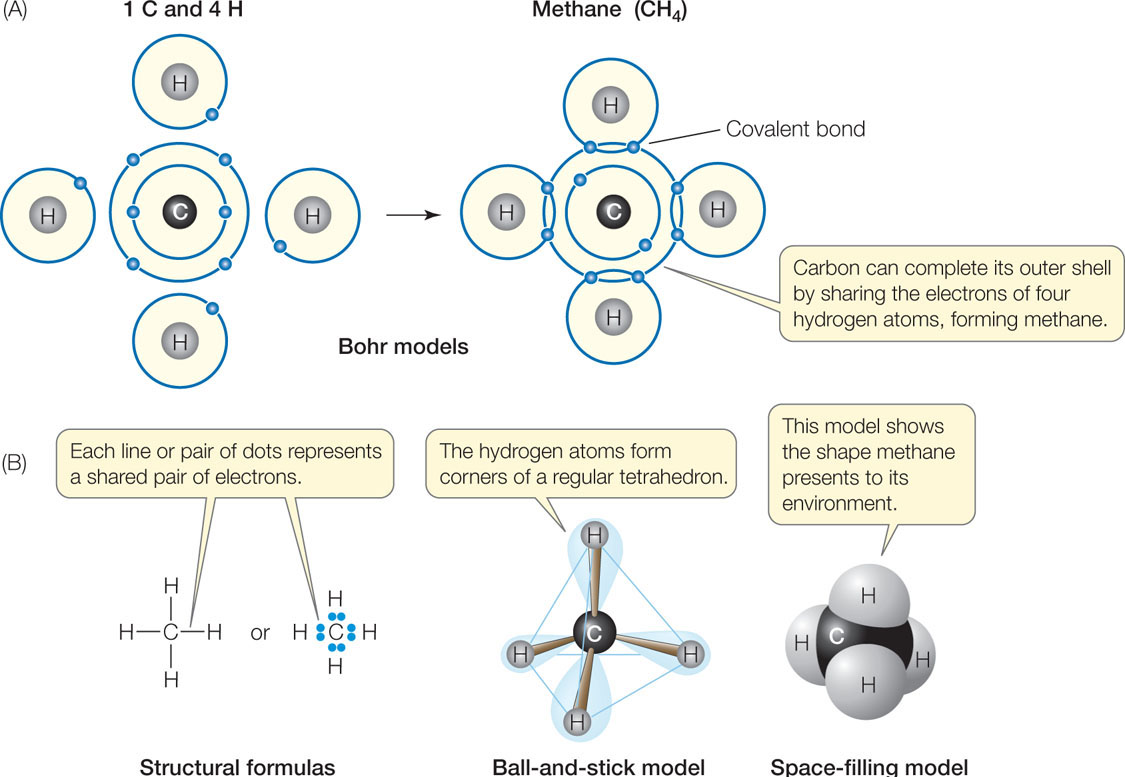
![[Best Answer] How many covalent bonds does carbon form if each of its](https://us-static.z-dn.net/files/df7/e45f4693a936673d74acd687e2bb88cf.png)