Why Do Noble Gases Rarely Form Bonds With Other Atoms - Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. Web in chemistry, the noble gases are stable and seldom react to other chemical elements. Noble gases almost always exist as single. Explore why noble gases don't bond, learn that they are non. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact. Noble gases can also form endohedral fullerene. The noble gases have full outer shells of electrons, and so cannot share other atoms’ electrons to form. Web the elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely. Web the noble gases are colourless, odourless, tasteless, nonflammable gases.
What's So Noble About Noble Gases? Owlcation Education
Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Because they’re reluctant to share electrons from their. Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. Web the elements belonging to the noble gases, including neon and helium,.
PPT Reading the Periodic Table PowerPoint Presentation, free download
Explore why noble gases don't bond, learn that they are non. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact. Web a noble gas atom has a completely full outer shell of electrons. Web the noble gases are colourless, odourless,.
Chemical Bonding
Explore why noble gases don't bond, learn that they are non. Atoms of these elements (e.g., helium,. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Web a noble gas atom has a completely full outer shell of electrons. Web instead, one might describe the noble gases as aloof.
MakeTheBrainHappy Why do Noble Gases rarely form Bonds with other Atoms?
Web instead, one might describe the noble gases as aloof. Web the noble gases are colourless, odourless, tasteless, nonflammable gases. Web what amounts to a constant pursuit for humans just comes naturally to noble gases. Web the theory of chemical bonding explained why. Noble gases can also form endohedral fullerene.
PPT Chapter 7 PowerPoint Presentation, free download ID6909184
Web the theory of chemical bonding explained why. Atoms of these elements (e.g., helium,. Because they’re reluctant to share electrons from their. Web when 2 atoms come close enough, the nucleus and the electron cloud attract each other, the electron clouds are. Web a noble gas atom has a completely full outer shell of electrons.
Formation of Ions and Ionic Compounds Good Science
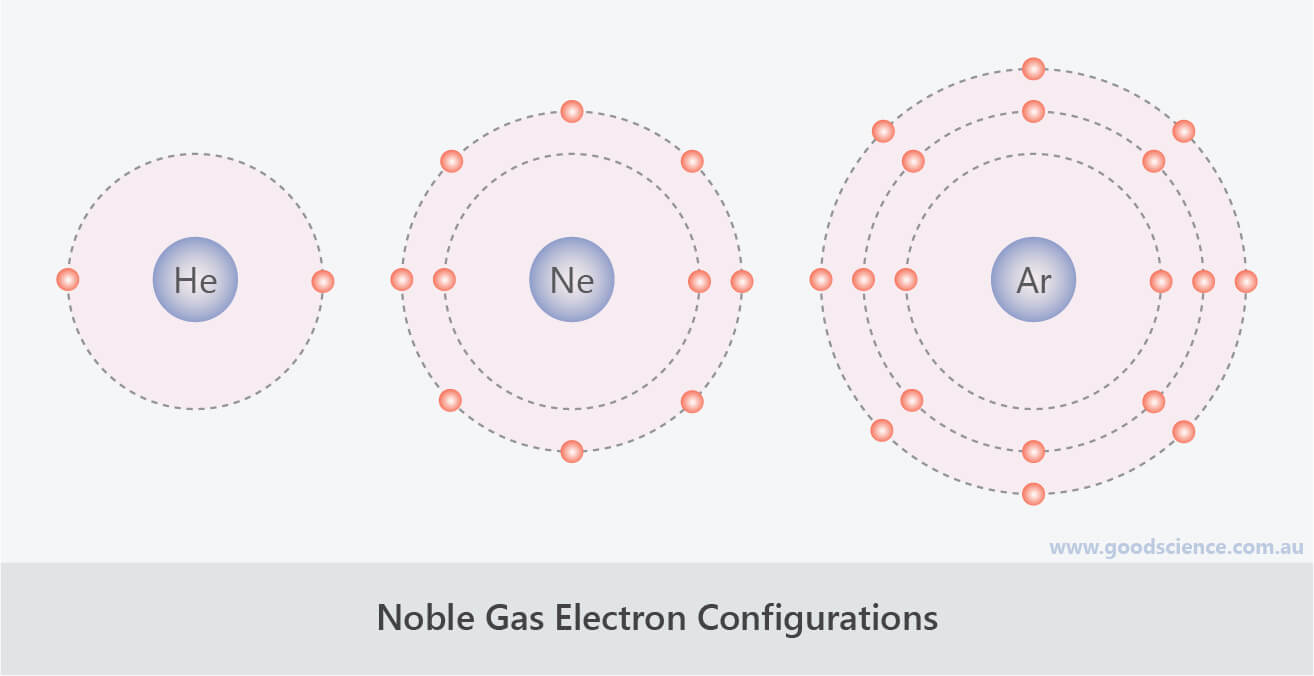
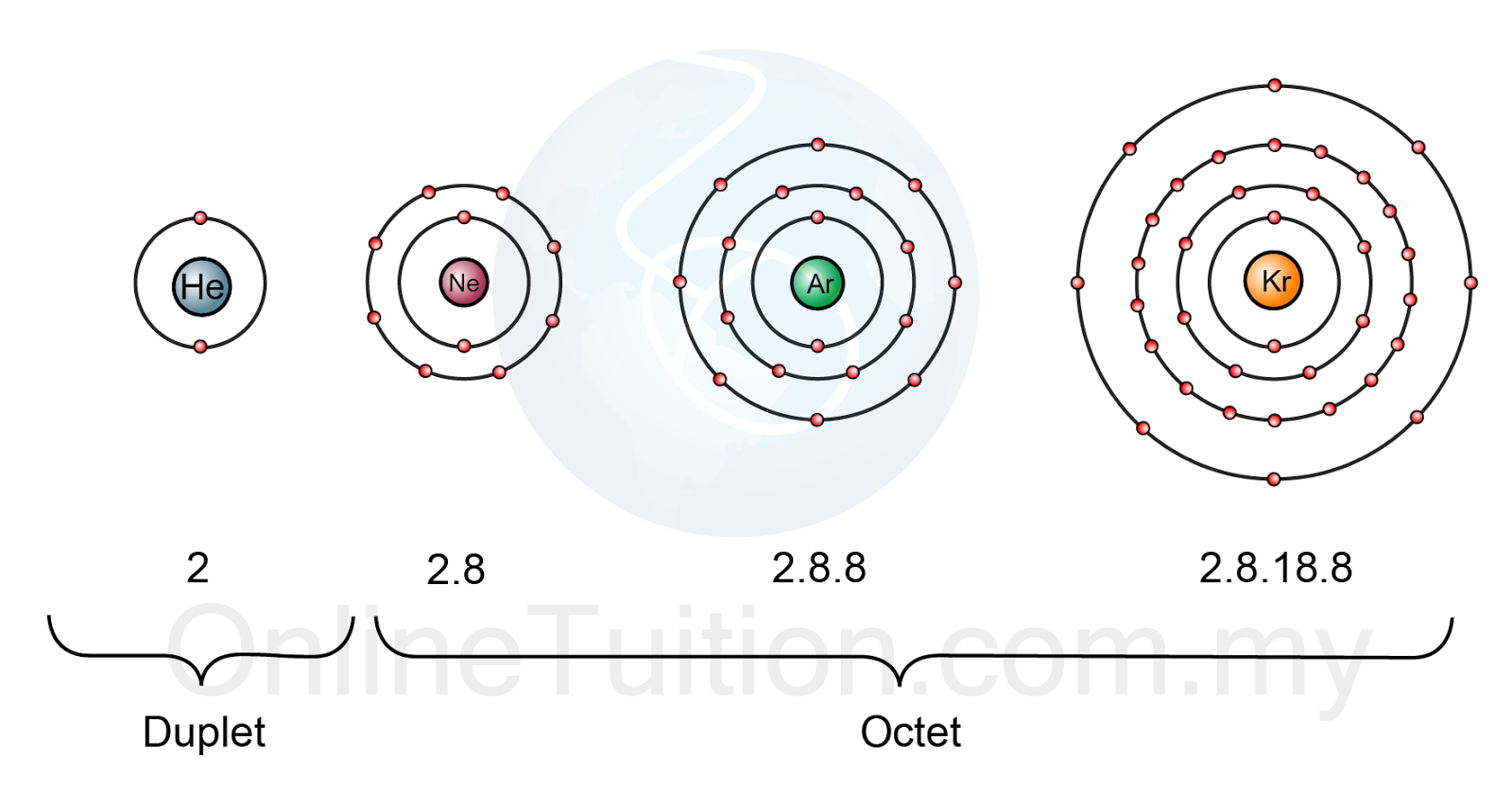
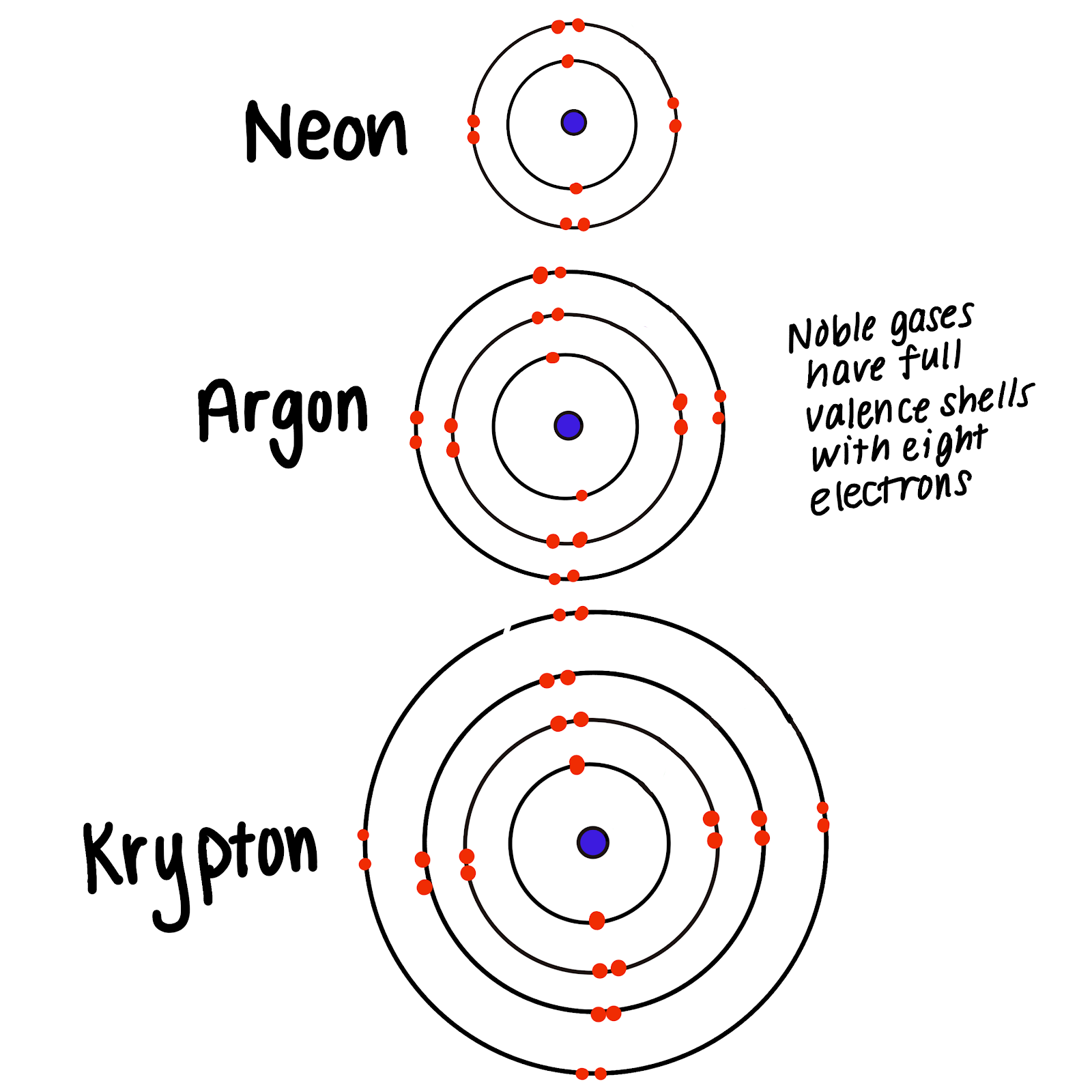
Web noble gases' outer shells are already filled with 8 electrons (other than he, which has 2, but is still filled and stable). Explore why noble gases don't bond, learn that they are non. Because they’re reluctant to share electrons from their. Web when 2 atoms come close enough, the nucleus and the electron cloud attract each other, the electron.
Stability of Noble Gases SPM Chemistry
Noble gases can also form endohedral fullerene. Because they’re reluctant to share electrons from their. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Atoms of these elements (e.g., helium,.
Why Don't Noble Gases Bond? Video & Lesson Transcript
Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Noble gases can also form endohedral fullerene. Web what amounts to a constant pursuit for humans just comes naturally to noble gases. Web.
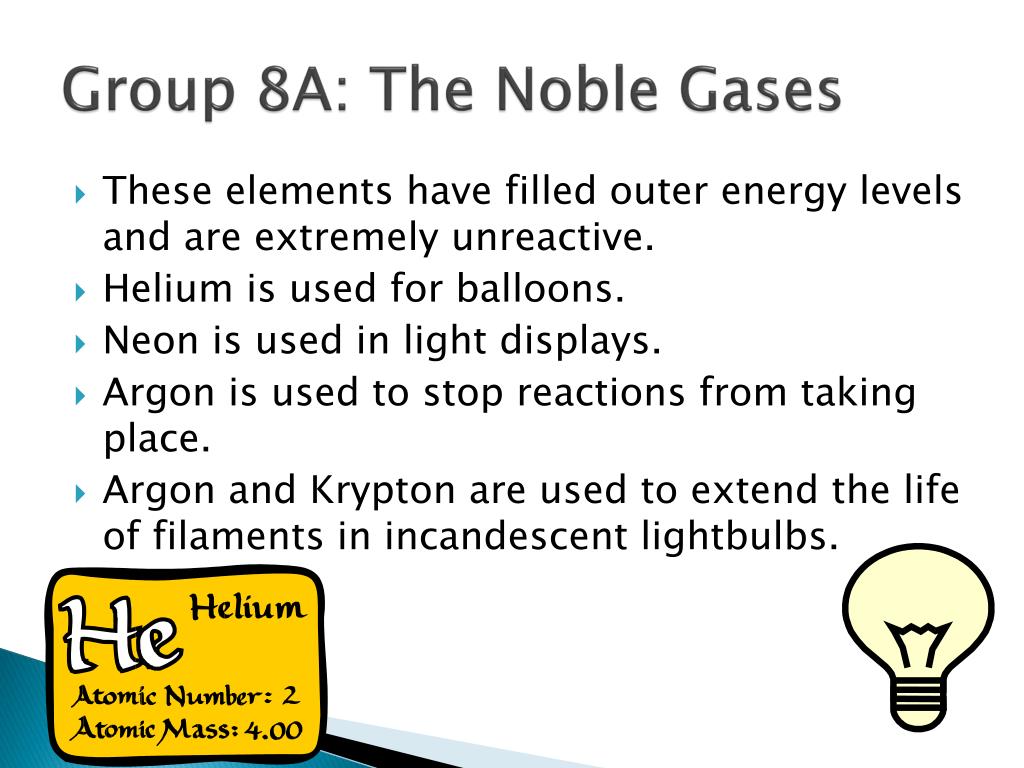
Group 18 The Noble Gases
Web noble gases' outer shells are already filled with 8 electrons (other than he, which has 2, but is still filled and stable). Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form. Atoms of these elements (e.g., helium,. Web the atoms of noble gases already have complete outer shells, so they.
The groups, and electron dot diagrams Presentation Chemistry
Web what amounts to a constant pursuit for humans just comes naturally to noble gases. Web the noble gases are colourless, odourless, tasteless, nonflammable gases. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web the bonding in water creates dipoles that attract other water molecules in ways that two atoms.
Atoms of these elements (e.g., helium,. Noble gases almost always exist as single. Web the elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely. Web the theory of chemical bonding explained why. Noble gases can also form endohedral fullerene. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms. Web what amounts to a constant pursuit for humans just comes naturally to noble gases. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form. Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons. Because they’re reluctant to share electrons from their. Web in chemistry, the noble gases are stable and seldom react to other chemical elements. Web the bonding in water creates dipoles that attract other water molecules in ways that two atoms of neon don't. Explore why noble gases don't bond, learn that they are non. Web instead, one might describe the noble gases as aloof. Web when 2 atoms come close enough, the nucleus and the electron cloud attract each other, the electron clouds are. The noble gases have full outer shells of electrons, and so cannot share other atoms’ electrons to form. Web the noble gases are colourless, odourless, tasteless, nonflammable gases. Web a noble gas atom has a completely full outer shell of electrons. Web the noble gases (group 18) are located in the far right of the periodic table and were previously referred to as the inert gases due to the fact. Web noble gases' outer shells are already filled with 8 electrons (other than he, which has 2, but is still filled and stable).
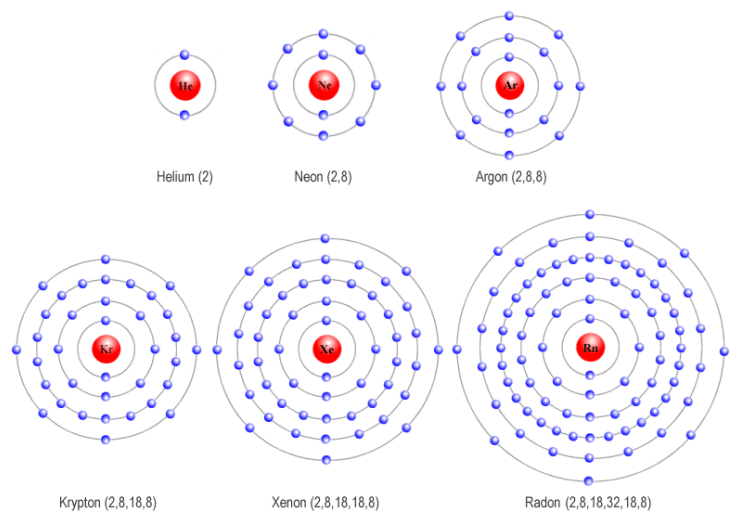
The Noble Gases Have Full Outer Shells Of Electrons, And So Cannot Share Other Atoms’ Electrons To Form.
Web this group has been referred to as the “inert” gases, indicating that they are chemically inert, or unreactive. Web instead, one might describe the noble gases as aloof. Web in chemistry, the noble gases are stable and seldom react to other chemical elements. Web noble gases have a full valence shell, which is why they rarely form bonds with other atoms.
Web A Noble Gas Atom Has A Completely Full Outer Shell Of Electrons.
Web the noble gases are colourless, odourless, tasteless, nonflammable gases. Atoms of these elements (e.g., helium,. Web what amounts to a constant pursuit for humans just comes naturally to noble gases. Web the atoms of noble gases already have complete outer shells, so they have no tendency to lose, gain, or share electrons.
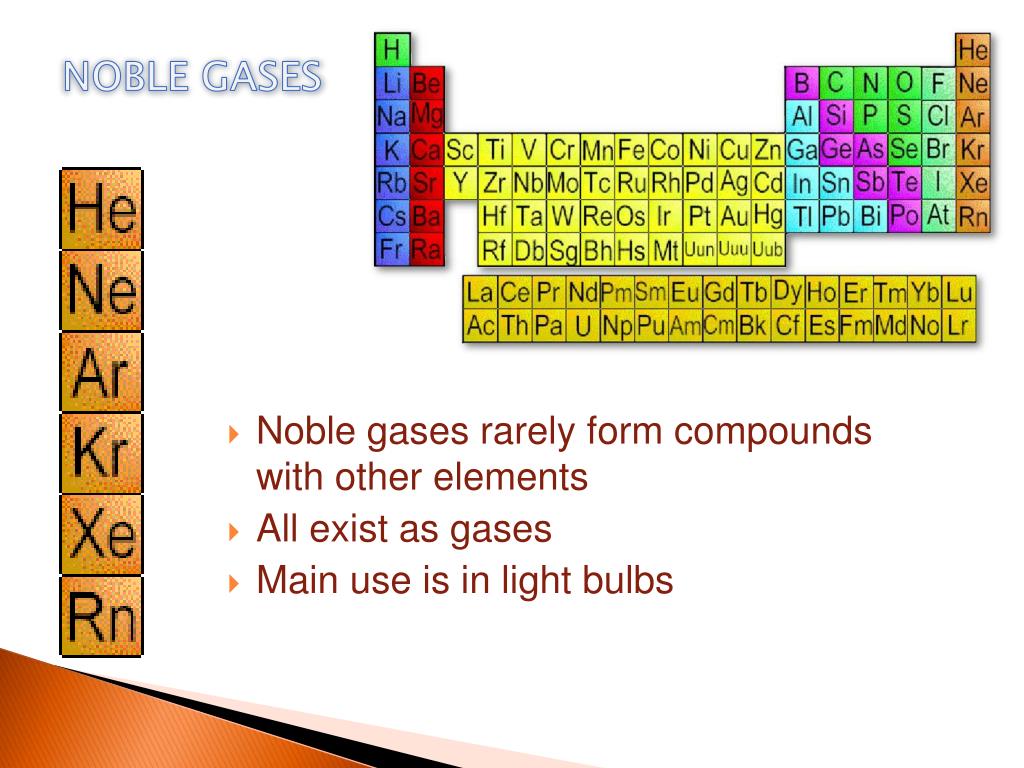
Noble Gases Can Also Form Endohedral Fullerene.
Web noble gases' outer shells are already filled with 8 electrons (other than he, which has 2, but is still filled and stable). Explore why noble gases don't bond, learn that they are non. Web the bonding in water creates dipoles that attract other water molecules in ways that two atoms of neon don't. Noble gases almost always exist as single.
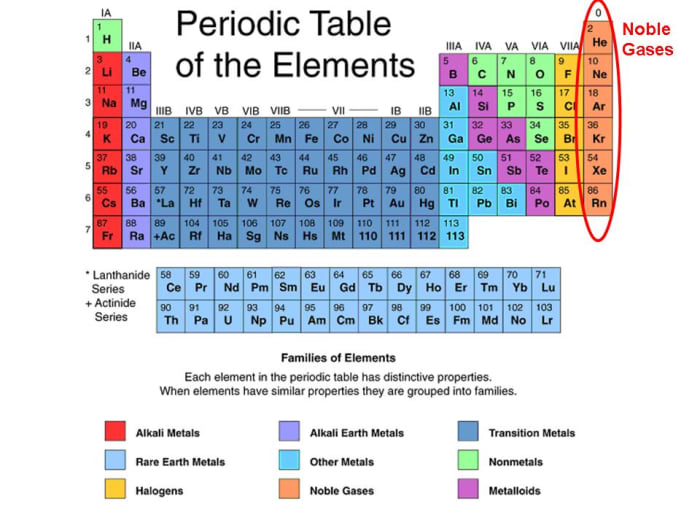
Web The Noble Gases (Group 18) Are Located In The Far Right Of The Periodic Table And Were Previously Referred To As The Inert Gases Due To The Fact.
Because they’re reluctant to share electrons from their. Web the full valence electron shells of these atoms make noble gases extremely stable and unlikely to form. Web when 2 atoms come close enough, the nucleus and the electron cloud attract each other, the electron clouds are. Web the elements belonging to the noble gases, including neon and helium, have atoms with full outer shells and rarely.



.PNG)