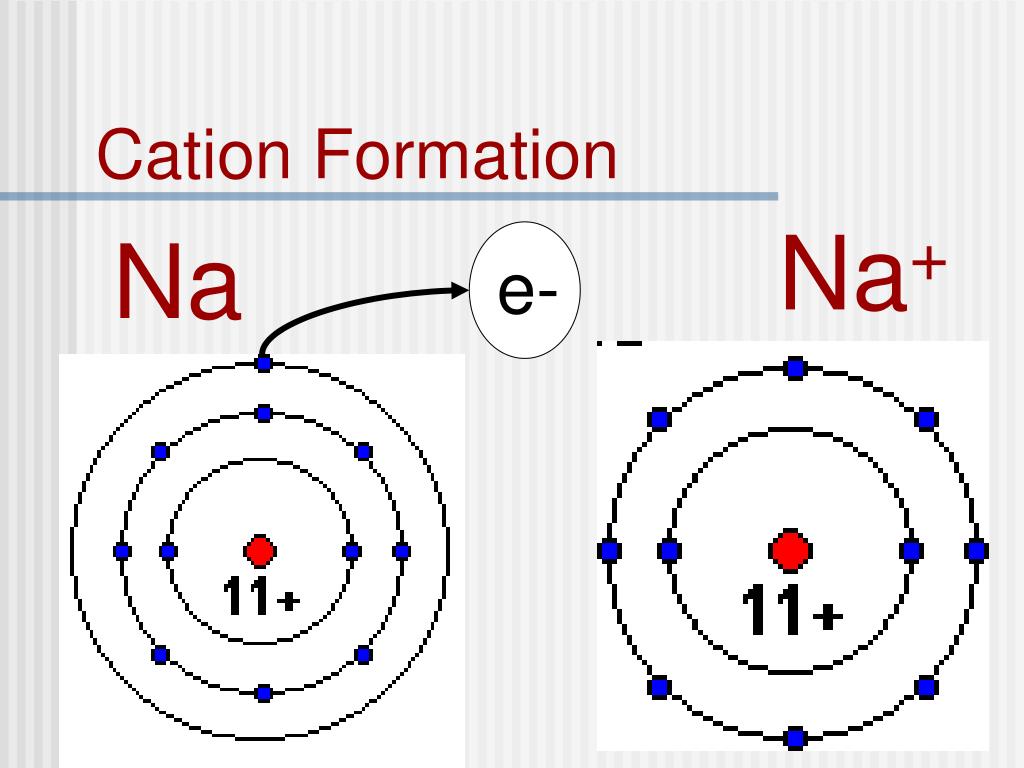
Why Do Metals Form Cations - Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to. For example, the alkali metals and the alkaline earth metals. Web they are formed when a metal loses its electrons. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. Metals generally have 1,2 , or 3 valence electrons which can be lost to attain octet configuration and thus they. Web metal ions in aqueous solution. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are.
Do You Know How to Tell Cation and Anion Ions Apart? Chemistry
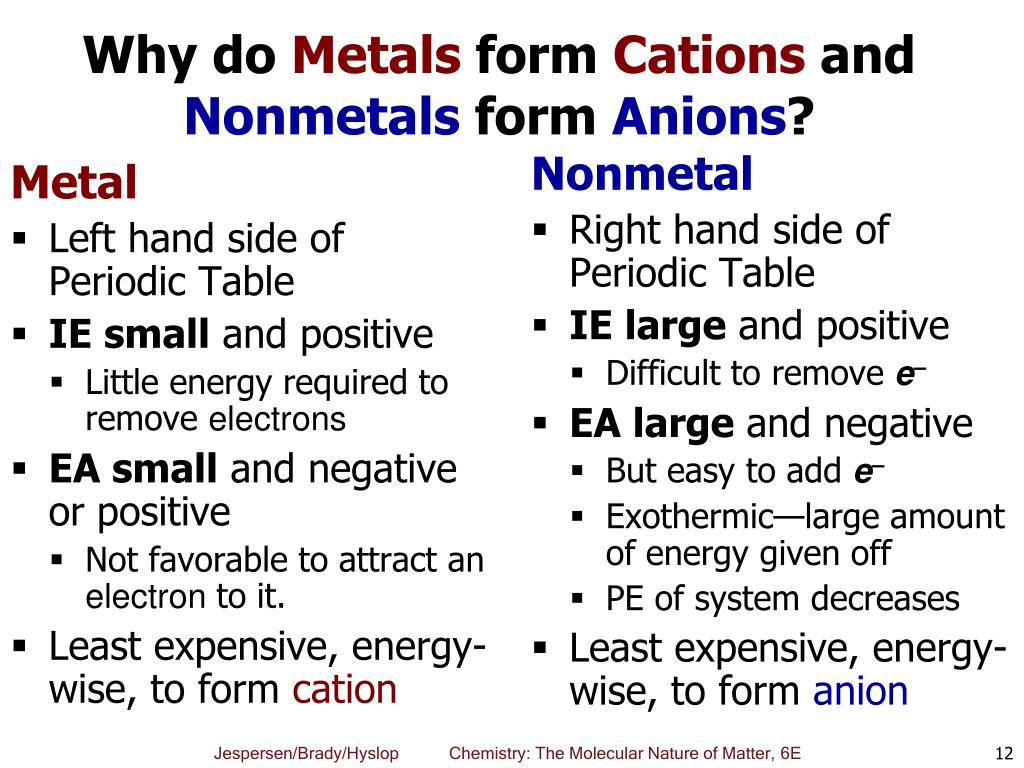
Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. It has fewer electrons than protons. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. Web elements that are metals form cations. They absorb energy (endothermic) to lose.
17 Best images about What is an ion? on Pinterest Models, Shape and
Metals like to lose valence electrons to form cations to have a fully stable octet. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are. Bismuth, mercury and iron are also poor conductors density: A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of.
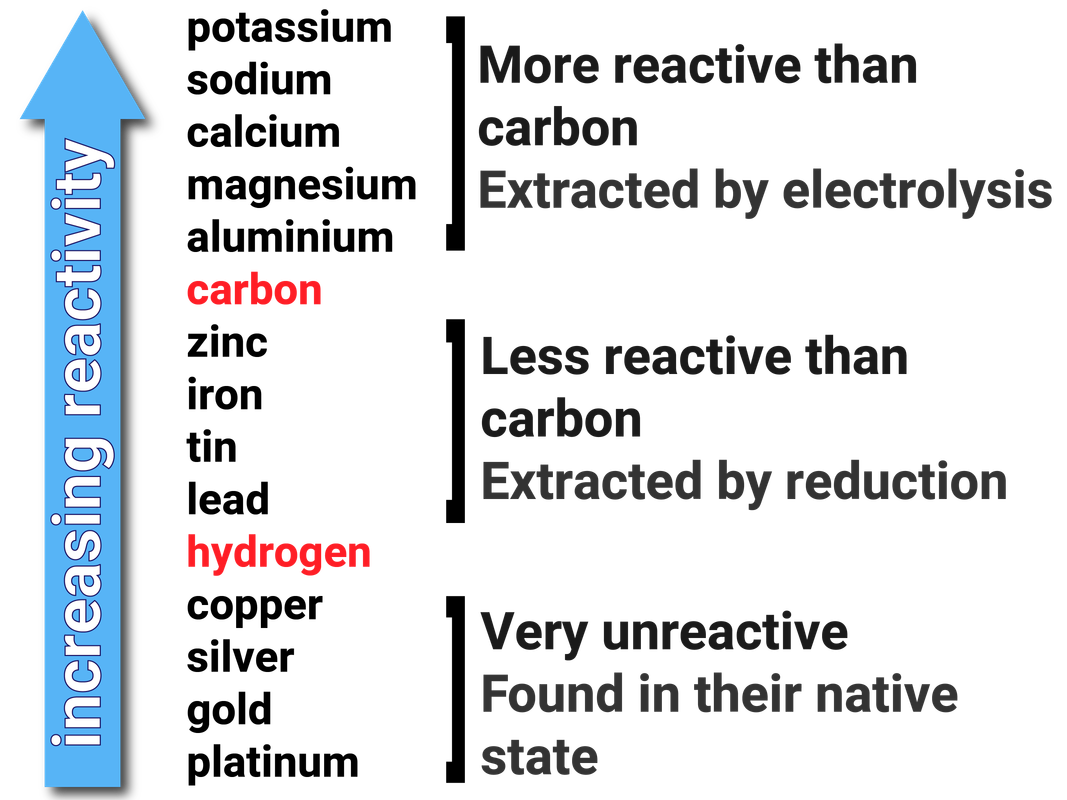
Obtaining and Using Metals Edexcel T4 revisechemistry.uk
They lose one or more than one electron. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to. Web so far, we have considered elements that typically.
Chem matters ch6_ionic_bond
Web they are formed when a metal loses its electrons. Web so far, we have considered elements that typically form cations of one particular charge. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to. Web atoms that lose electrons acquire a positive charge as a result because.
PPT 1 Name the ions formed by these elements and classify them as
Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are. Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. The ions formed are negative,. Web they are formed when a metal loses its electrons. It has fewer.
PPT Ionic Bonding PowerPoint Presentation, free download ID5679234
Bismuth, mercury and iron are also poor conductors density: The ions formed are negative,. Web elements that are metals form cations. They absorb energy (endothermic) to lose. It has fewer electrons than protons.
PPT ChemCatalyst PowerPoint Presentation, free download ID5516040
In the periodic table, note that the metallic elements are in group i, group ii. They absorb energy (endothermic) to lose. Web they are formed when a metal loses its electrons. The ions formed are negative,. Web lead is the poorest conductor of heat.
PPT The Nomenclature of Binary Compounds PowerPoint Presentation ID
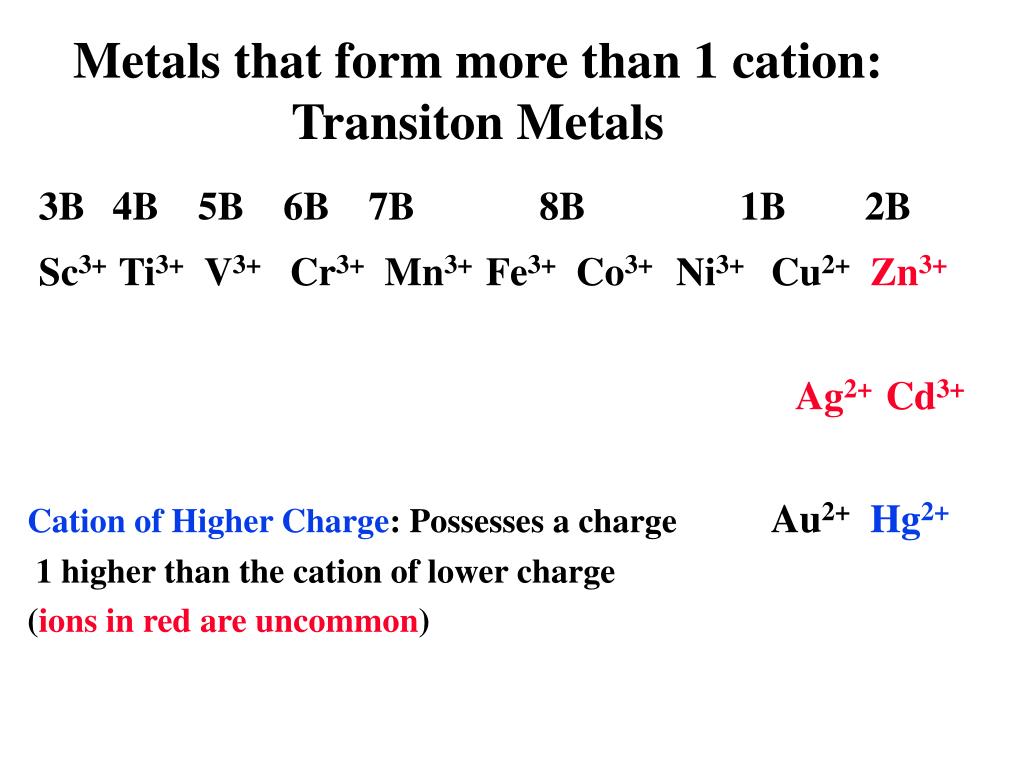
In the periodic table, note that the metallic elements are in group i, group ii. Web metal ions in aqueous solution. Ions form primarily through loss of s electrons. Web summary transition metals have unfilled inner d electron shells. They absorb energy (endothermic) to lose.
PPT Chapter 9 The Basics of Chemical Bonding PowerPoint Presentation
Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. Web metal ions in aqueous solution. Web lead is the poorest conductor of heat. Metals like to.
PPT Naming IONS & formulas for Ionic Compounds PowerPoint
Web summary transition metals have unfilled inner d electron shells. Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. The ions formed are negative,. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to. Web they are formed.
Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. Metals like to lose valence electrons to form cations to have a fully stable octet. Web lead is the poorest conductor of heat. They lose one or more than one electron. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. Web they are formed when a metal loses its electrons. For example, the alkali metals and the alkaline earth metals. Web because of their low positive charge (+1) and relatively large ionic radii, alkali metal cations have only a weak tendency to. Metals generally have 1,2 , or 3 valence electrons which can be lost to attain octet configuration and thus they. In the periodic table, note that the metallic elements are in group i, group ii. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m. Bismuth, mercury and iron are also poor conductors density: The ions formed are negative,. Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. Web forming positive ions (cations) atoms lose electrons from their outer shell when they form positive ions, called cations. Web metal ions in aqueous solution. Web summary transition metals have unfilled inner d electron shells. Web so far, we have considered elements that typically form cations of one particular charge. Web elements that are metals form cations.
Web Cations Are Atoms That Contain A Positive Charge, And They Are Formed When The Atoms Lose Electrons Which Are.
They absorb energy (endothermic) to lose. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. A metal ion in aqueous solution or aqua ion is a cation, dissolved in water, of chemical formula [m. In the periodic table, note that the metallic elements are in group i, group ii.
Web Because Of Their Low Positive Charge (+1) And Relatively Large Ionic Radii, Alkali Metal Cations Have Only A Weak Tendency To.
Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. It has fewer electrons than protons. Metals like to lose valence electrons to form cations to have a fully stable octet. Web metallic solids are composed of metal cations held together by a delocalized sea of valence electrons.
Web Elements That Are Metals Form Cations.
Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. They lose one or more than one electron. Web metal ions in aqueous solution. Bismuth, mercury and iron are also poor conductors density:
Ions Form Primarily Through Loss Of S Electrons.
Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy,. Web lead is the poorest conductor of heat. Web so far, we have considered elements that typically form cations of one particular charge. The ions formed are negative,.