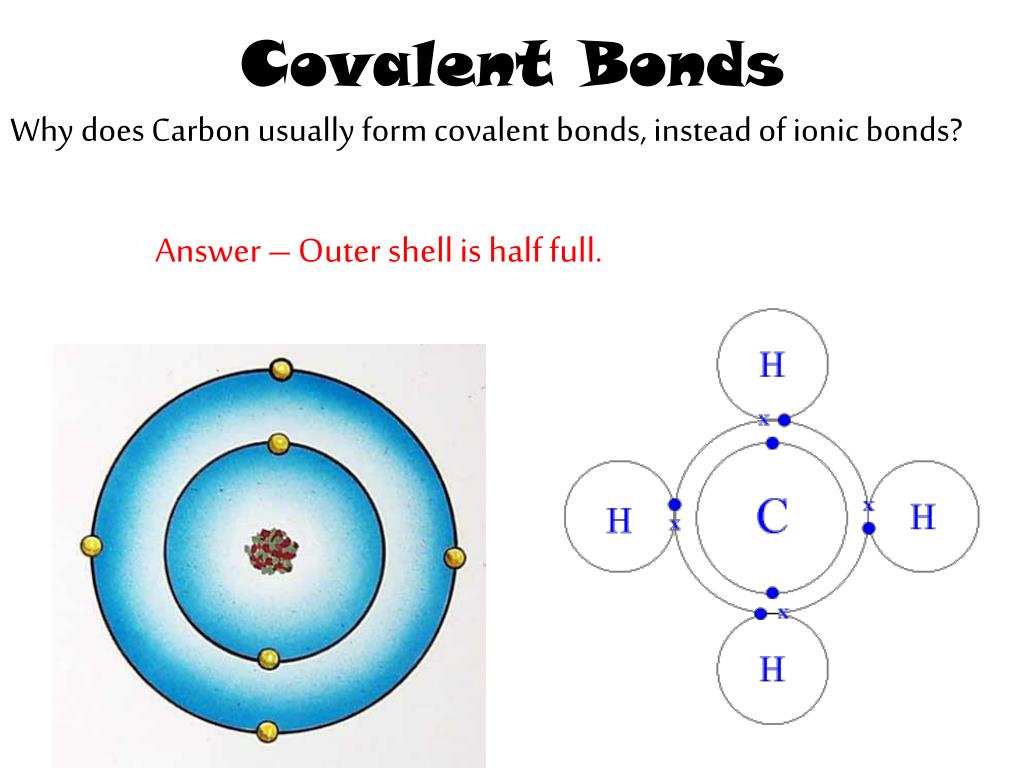
Why Do Carbon Form Covalent Bond - Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the. Web covalent bonds are especially important since most carbon molecules interact primarily through covalent. Web by anne marie helmenstine, ph.d. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with. Chemical bonds between nonmetals are known as covalent bonds. Web why does carbon form covalent bonds? Web why do carbon form only covalent bonds? Web it cannot lose 4 electrons as it involves a lot of energy.
Chapter 8 Covalent Bonding Covalent bonding Usually forms
Solution carbon carbon has the unique property of catenation (forming a long. Web two reasons for large number of carbon compounds: Simple molecules, which contain a fixed number of atoms. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon. A covalent bond forms when the bonded atoms have.
Why does Carbon always form Covalent Bond? Freakgenie
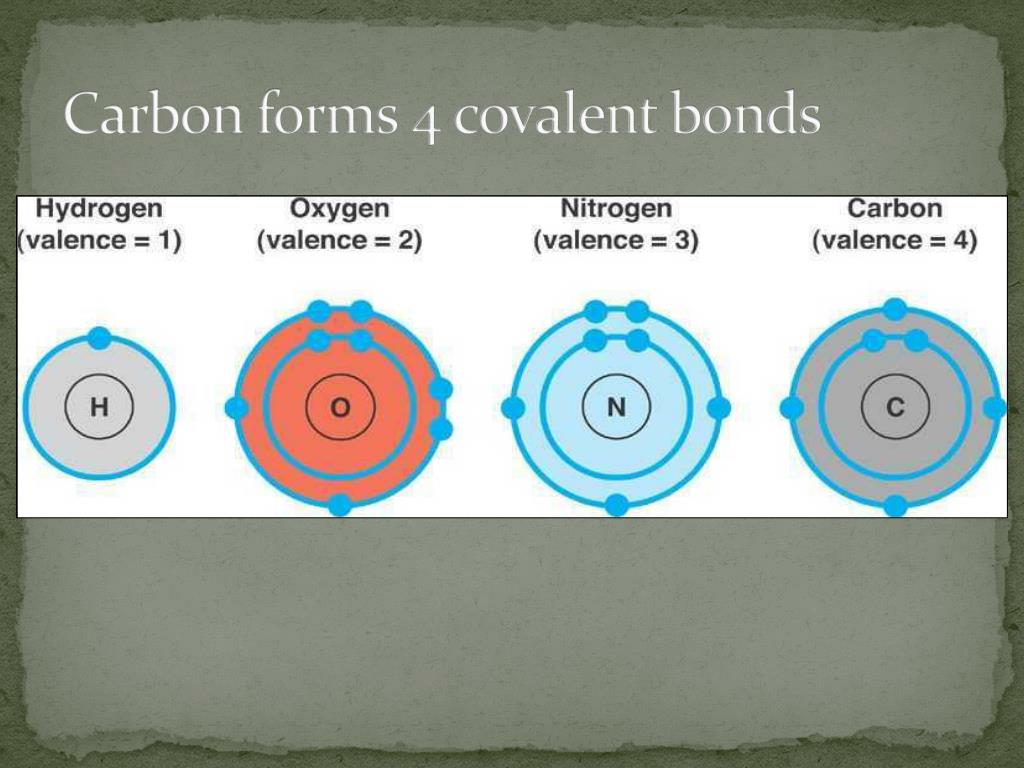
It has an electronic configuration 1 s 2 2 s 2 2 p 2. Web by anne marie helmenstine, ph.d. Web covalent bonds are especially important since most carbon molecules interact primarily through covalent. Web covalent bond helps in the formation of compounds with the help of carbon. The electrons involved are in the outer shells.
PPT Unit 1 Biochemistry The Chemistry of Life PowerPoint
A covalent bond forms when the bonded atoms have a. Pm expert answer hi, carbon. Web four covalent bonds can be formed by carbon. The carbon atom is unique among elements in its tendency to form extensive networks of covalent bonds not only with. It has an electronic configuration 1 s 2 2 s 2 2 p 2.
[Best Answer] How many covalent bonds does carbon form if each of its
Solution carbon carbon has the unique property of catenation (forming a long. For any atom, stability is achieved by following the. A covalent bond forms when the bonded atoms have a. Web covalent bond helps in the formation of compounds with the help of carbon. Simple molecules, which contain a fixed number of atoms.
PPT Carbon Compounds PowerPoint Presentation, free download ID2319022
For any atom, stability is achieved by following the. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon. Web four covalent bonds can be formed by carbon. Web covalent bonds are especially important since most carbon molecules interact primarily through covalent. Web by anne marie helmenstine, ph.d.
[Solved] Carbon Dioxide CO2 Ionic or Covalent Bond? Ultimate Guide
It is because carbon has an electronic. Web by anne marie helmenstine, ph.d. It has an electronic configuration 1 s 2 2 s 2 2 p 2. For any atom, stability is achieved by following the. Simple molecules, which contain a fixed number of atoms.
Four covalent bonds. Carbon has four valence electrons and here a
Web carbon can form four covalent bonds. Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the. A covalent bond forms when the bonded atoms have a. Updated on july 28, 2019 carbon and.
PPT 2.1 Nature of Matter PowerPoint Presentation, free download ID
Diamond is a giant covalent substance in which: Web covalent bond helps in the formation of compounds with the help of carbon. Pm expert answer hi, carbon. Asked by venkat34 | 05 jan, 2016, 06:44: The unique ability of carbon to form bonds with other atoms.
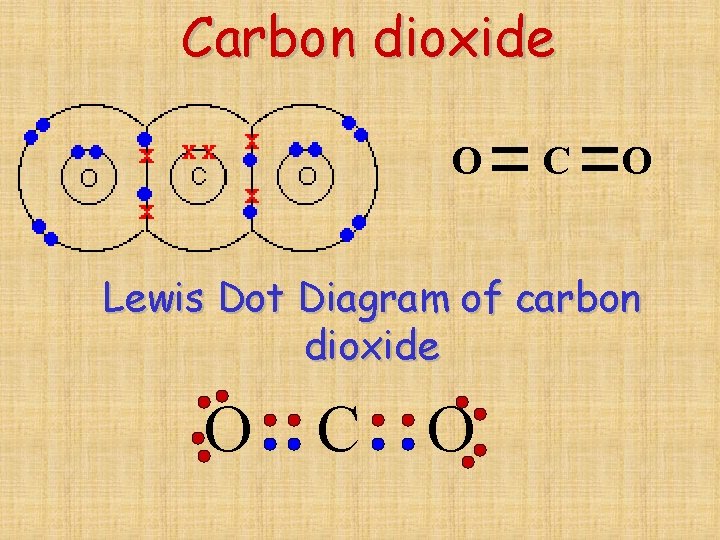
How many convalent bonds are there in one molecule of carbon dioxide
Due to the number of electrons present in the last shell, carbon forms covalent compounds. The most common form is the single bond: Solution carbon carbon has the unique property of catenation (forming a long. Web why does carbon form covalent bonds? The unique ability of carbon to form bonds with other atoms.
PPT Carbohydrates, Lipids and Nucleic Acids PowerPoint Presentation
It is because carbon has an electronic. Web carbon can form four covalent bonds. Covalent bonds are bonds that are formed between nonmetals. Web why does carbon form covalent bonds? In most cases, carbon shares electrons with other atoms.
Web why do carbon form only covalent bonds? Two atoms share a pair of. Web if carbon forms 4 bonds rather than 2, twice as much energy is released and so the resulting molecule becomes even. In most cases, carbon shares electrons with other atoms. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the. It is because carbon has an electronic. Solution carbon carbon has the unique property of catenation (forming a long. Due to the number of electrons present in the last shell, carbon forms covalent compounds. Chemical bonds between nonmetals are known as covalent bonds. Each carbon atom is joined to four other carbon atoms. The most common form is the single bond: The unique ability of carbon to form bonds with other atoms. Web the binding arises from the electrostatic attraction of their nuclei for the same electrons. Web two reasons for large number of carbon compounds: It has an electronic configuration 1 s 2 2 s 2 2 p 2. Web carbon generally forms compounds by covalent bonds because: Updated on july 28, 2019 carbon and its bonds are key to organic chemistry and biochemistry as well as general. Pm expert answer hi, carbon. Covalent bonds are bonds that are formed between nonmetals. A covalent bond forms when the bonded atoms have a.
Chemical Bonds Between Nonmetals Are Known As Covalent Bonds.
It has an electronic configuration 1 s 2 2 s 2 2 p 2. Each carbon atom is joined to four other carbon atoms. Web two reasons for large number of carbon compounds: Two atoms share a pair of.
Due To The Number Of Electrons Present In The Last Shell, Carbon Forms Covalent Compounds.
Updated on july 28, 2019 carbon and its bonds are key to organic chemistry and biochemistry as well as general. The electrons involved are in the outer shells. Web the binding arises from the electrostatic attraction of their nuclei for the same electrons. Also, it cannot gain 4 electrons because the nucleus cannot hold on to the.
Solution Carbon Carbon Has The Unique Property Of Catenation (Forming A Long.
Diamond is a giant covalent substance in which: The unique ability of carbon to form bonds with other atoms. Web carbon generally forms compounds by covalent bonds because: Web four covalent bonds can be formed by carbon.
Web It Cannot Lose 4 Electrons As It Involves A Lot Of Energy.
Web carbon can form four covalent bonds. Asked by venkat34 | 05 jan, 2016, 06:44: Web covalent bond helps in the formation of compounds with the help of carbon. Web these four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in ccl 4 (carbon.



![[Best Answer] How many covalent bonds does carbon form if each of its](https://us-static.z-dn.net/files/df7/e45f4693a936673d74acd687e2bb88cf.png)

![[Solved] Carbon Dioxide CO2 Ionic or Covalent Bond? Ultimate Guide](http://www.bestfavy.com/wp-content/uploads/2021/06/Carbon-Dioxide-CO2-Ionic-or-Covalent-Bond1.jpg)