Why Are Halogens And Alkali Metals Likely To Form Ions - Because they are both just one electron short of having a. Web study with quizlet and memorize flashcards containing terms like how do positive ions and negative ions form?, why are halogens. Until the late 1960s there were few complexes of the alkali. Web therefore, halogens and alkali metals are the reactive elements that tend to lose one valence electron and gain one electron,. Well, the answer lies in the balance show. Web most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the. Web they only require one electron on as we talked about before, every element in canada really wants to have that noble gas. Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient. Web why are halogens and alkali metals likely to form ions? Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain.
ShowMe halogens
Because they are both just one electron short of having a. Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain. Alkali metals.
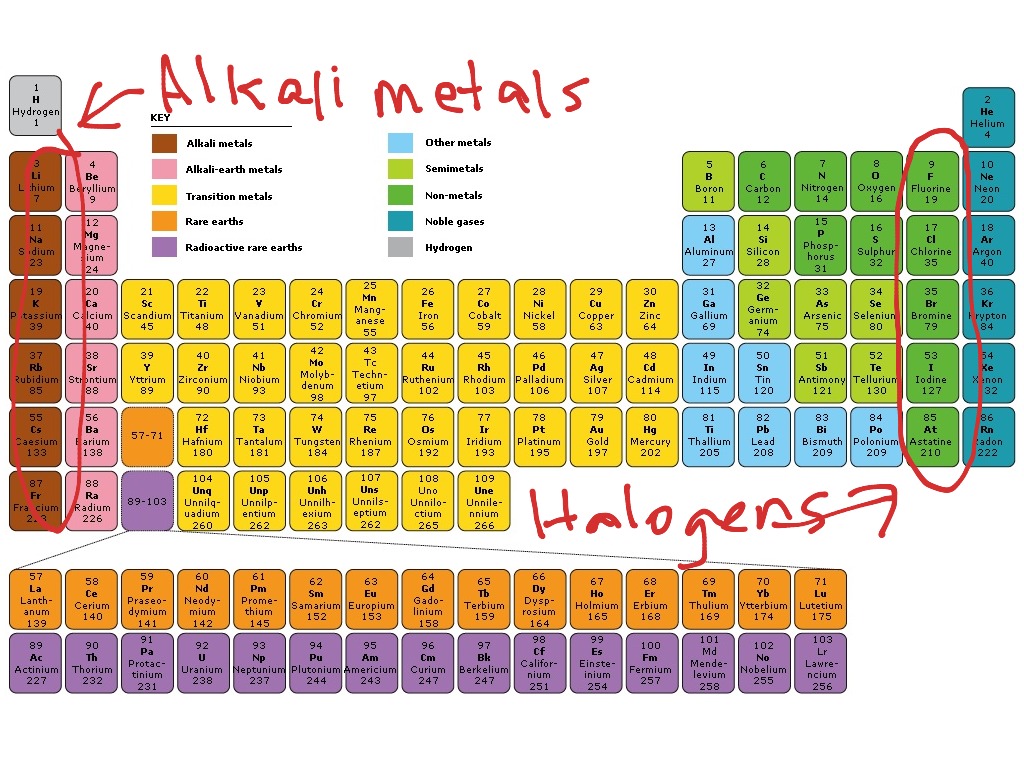
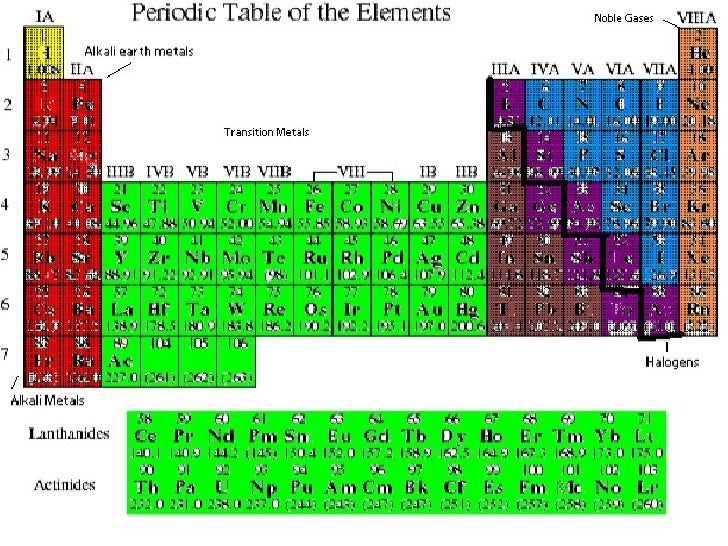
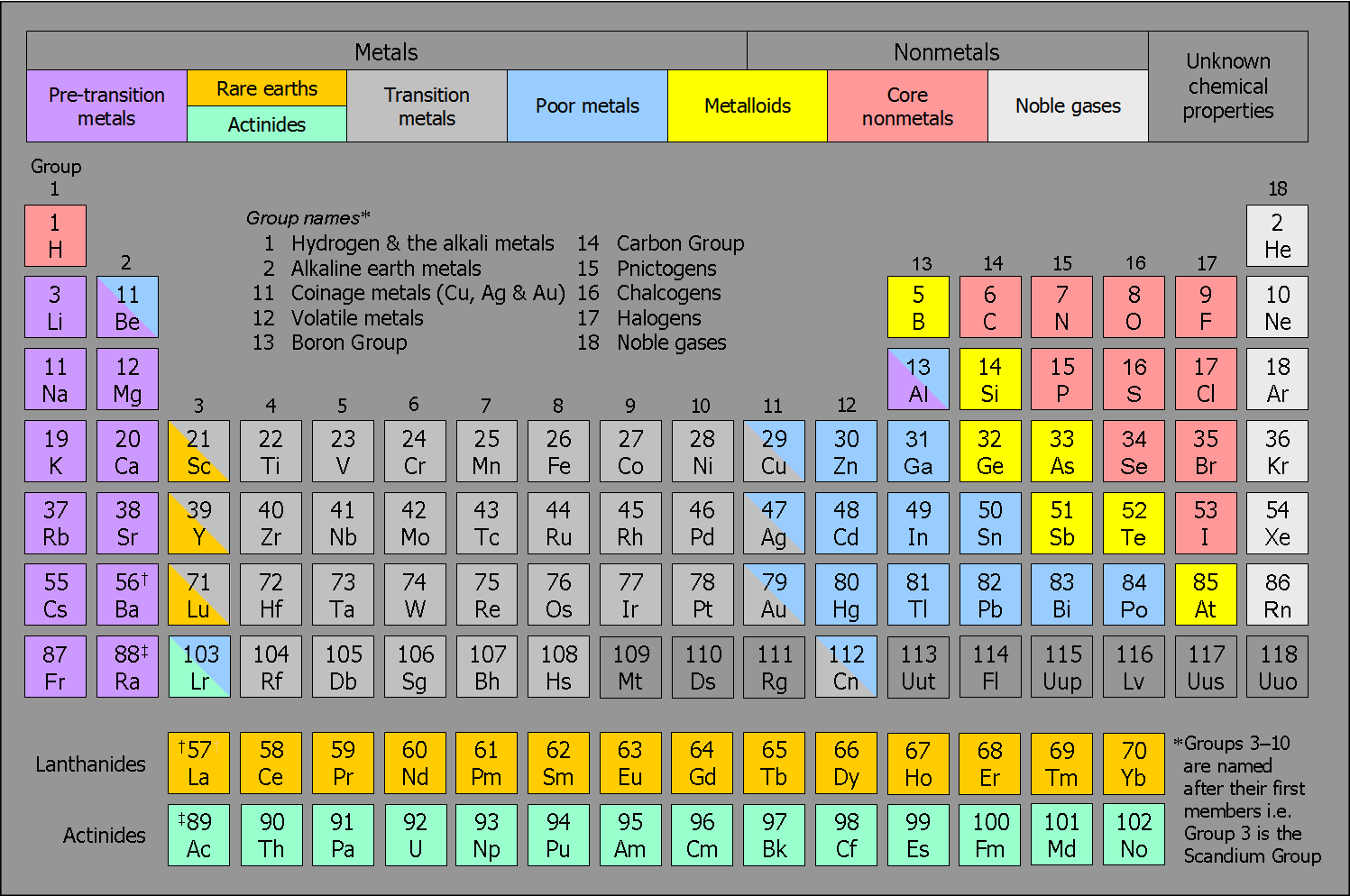
Periodic Table With Halogens Alkali Metals Periodic Table Timeline
Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain. Web halogens and alkali metals are likely to form ions because they both have low ionization energy as they only need to gain or lose. Web reaction with hydrogen all the halogens react directly with.
halogen Facts, Definition, Properties, & Uses Britannica
Web halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Web alkaline earth metals are always 2 + (lose both electrons in s subshell) transition metal ions do not follow an. Web anonymous libretexts learning objectives to describe how the alkali metals are isolated. So, as you know, alkaline metals are group.
The Halogens Alkali Metals And Alkaline Earth Have The Earth Images
Until the late 1960s there were few complexes of the alkali. Well, the answer lies in the balance show. To be familiar with the reactions,. Web answer (1 of 8): Web study with quizlet and memorize flashcards containing terms like how do positive ions and negative ions form?, why are halogens.
Why are halogens and alkali metals likely to form ions quizlet? Book Vea
Until the late 1960s there were few complexes of the alkali. Web they only require one electron on as we talked about before, every element in canada really wants to have that noble gas. Web halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Web therefore, halogens and alkali metals are the.
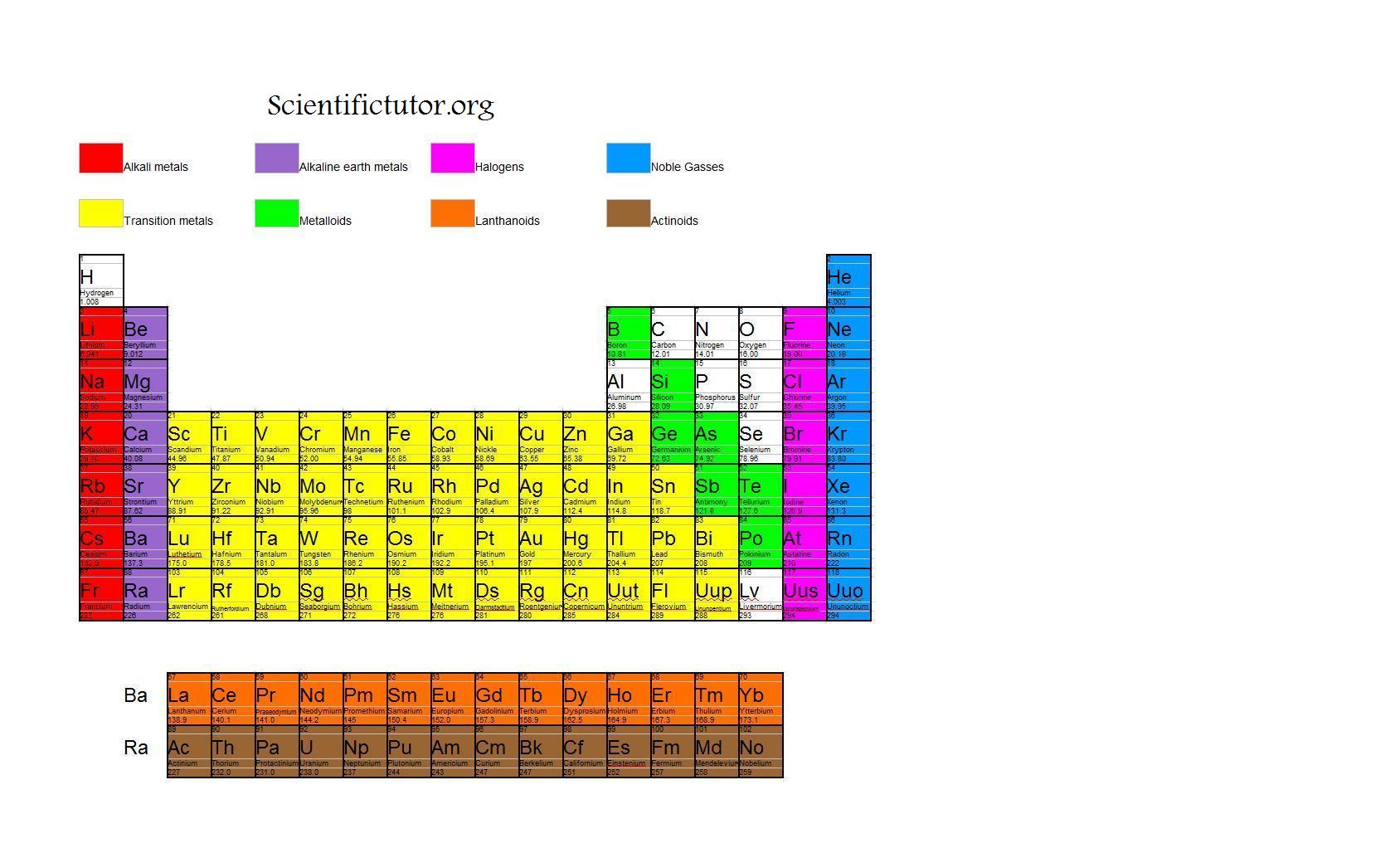
Chem Valence Electrons Scientific Tutor
Until the late 1960s there were few complexes of the alkali. Because they are both just one electron short of having a. Web halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table. Web why are halogens and alkali metals likely to form ions? Web all alkaline earth metals react with the halogens.
Alkaline Earth Metals Occurrence and Extraction,Physical Properties
Web so halogens tend to gain one electron to form a monovalent anion or negatively charged ion and achieve the. So, as you know, alkaline metals are group one, and they all. Web all alkaline earth metals react with the halogens to produce the corresponding halides, with oxygen to form the oxide (except for barium, which forms the. Web halogens.
Periodic Table With Halogens Alkali Metals Periodic Table Timeline
Web anonymous libretexts learning objectives to describe how the alkali metals are isolated. Web why are halogens and alkali metals likely to form ions? Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient. They react with metals to form metal halides, and with hydrogen to form acidic. Web most common nonmetallic substances such.
Periodic Table With Halogens Alkali Metals Periodic Table Timeline
Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient. Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas. Web study with quizlet and memorize flashcards containing terms like how do positive ions and negative ions form?, why are.
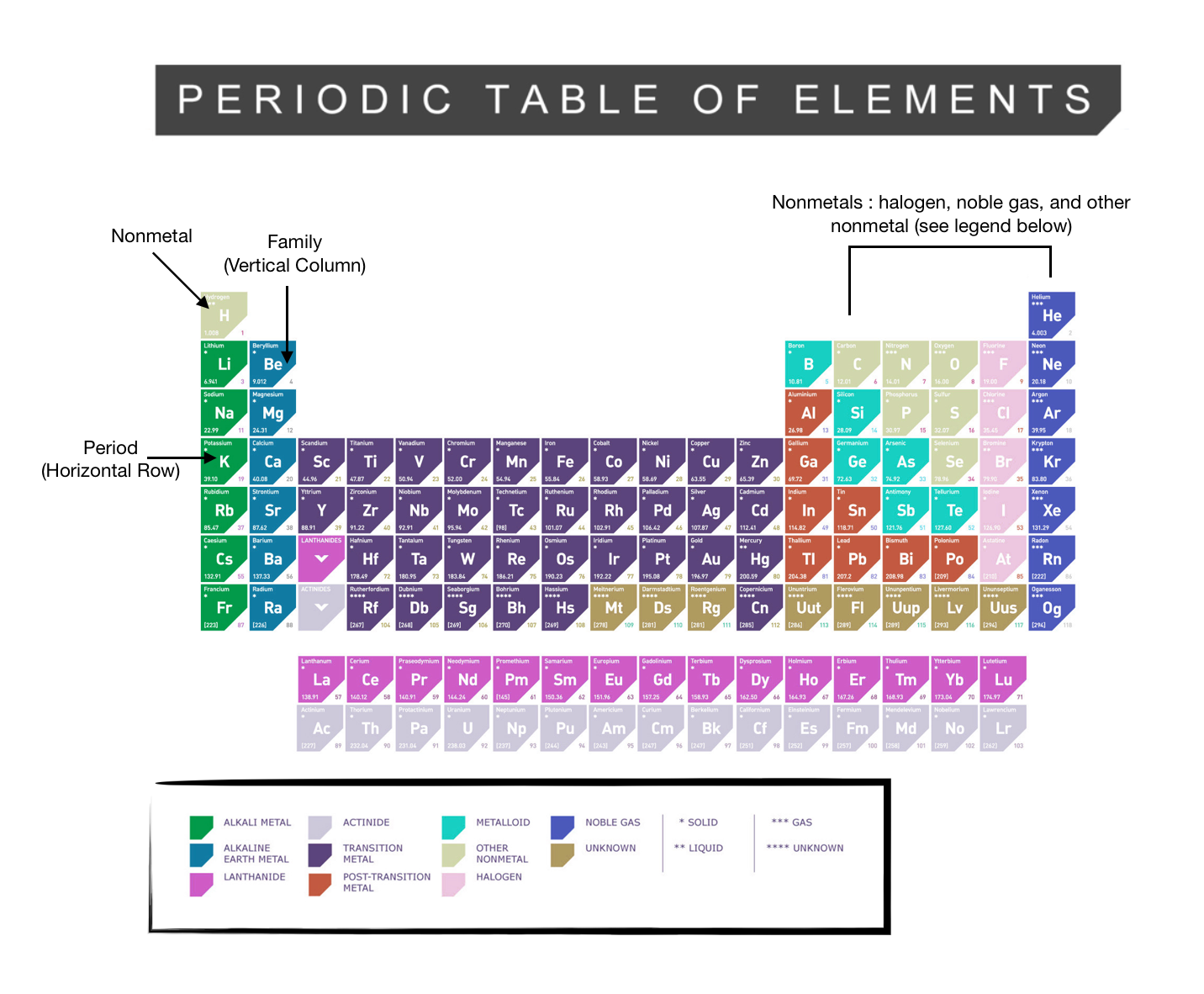
Periodic Table Alkali Metals Alkaline Earth Metals Halogens Noble Gases
Web most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the. Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain. Web all alkaline earth metals react with the halogens to produce the corresponding halides, with oxygen to form the.
Web when the alkali metals react with the different halogens (group 7 of the periodic table), the group of compounds formed are. Web therefore, halogens and alkali metals are the reactive elements that tend to lose one valence electron and gain one electron,. Web okay, so why are hella jin's and alkali metals likely the form ions? Web anonymous libretexts learning objectives to describe how the alkali metals are isolated. Because they are both just one electron short of having a. Web halogens need to gain only one electron and alkali metals need to lose one electron in order to reach the nearest noble gas. Web study with quizlet and memorize flashcards containing terms like how do positive ions and negative ions form?, why are halogens. Web why are halogens and alkali metals likely to form ions? Web answer (1 of 8): So, as you know, alkaline metals are group one, and they all. Expert solution step by step solved in 2 steps. Web so halogens tend to gain one electron to form a monovalent anion or negatively charged ion and achieve the. They react with metals to form metal halides, and with hydrogen to form acidic. Web introduction halogens form diatomic molecules (of the form x 2 , where x denotes a halogen atom) in their elemental states. Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient. Web why are halogens and alkali metals likely to form ions? Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain. Alkali metals will very happily donate their single outermost valence electron to form compounds with. Web they only require one electron on as we talked about before, every element in canada really wants to have that noble gas. Web halogen, any of the six nonmetallic elements that constitute group 17 (group viia) of the periodic table.
Web All Alkaline Earth Metals React With The Halogens To Produce The Corresponding Halides, With Oxygen To Form The Oxide (Except For Barium, Which Forms The.
Web why are halogens and alkali metals likely to form ions? Web introduction halogens form diatomic molecules (of the form x 2 , where x denotes a halogen atom) in their elemental states. They react with metals to form metal halides, and with hydrogen to form acidic. Web halogens in alkali metals are likely to form ions because their ionization energy is very low because they only need to lose or gain.
Web When The Alkali Metals React With The Different Halogens (Group 7 Of The Periodic Table), The Group Of Compounds Formed Are.
Until the late 1960s there were few complexes of the alkali. Alkali metals will very happily donate their single outermost valence electron to form compounds with. So, as you know, alkaline metals are group one, and they all. Well, the answer lies in the balance show.
Web Halogen, Any Of The Six Nonmetallic Elements That Constitute Group 17 (Group Viia) Of The Periodic Table.
Web most common nonmetallic substances such as halogens, halogen acids, sulfur, and phosphorus react with the. Web anonymous libretexts learning objectives to describe how the alkali metals are isolated. Web okay, so why are hella jin's and alkali metals likely the form ions? Web reaction with hydrogen all the halogens react directly with hydrogen, forming covalent bonds and—at sufficient.
Web Halogens In Alkali Metals Are Likely To Form Ions Because Their Ionization Energy Is Very Low Because They Only Need To Lose Or Gain.
Web why are halogens and alkali metals likely to form ions? Web therefore, halogens and alkali metals are the reactive elements that tend to lose one valence electron and gain one electron,. Web halogens and alkali metals are likely to form ions because they both have low ionization energy as they only need to gain or lose. Expert solution step by step solved in 2 steps.