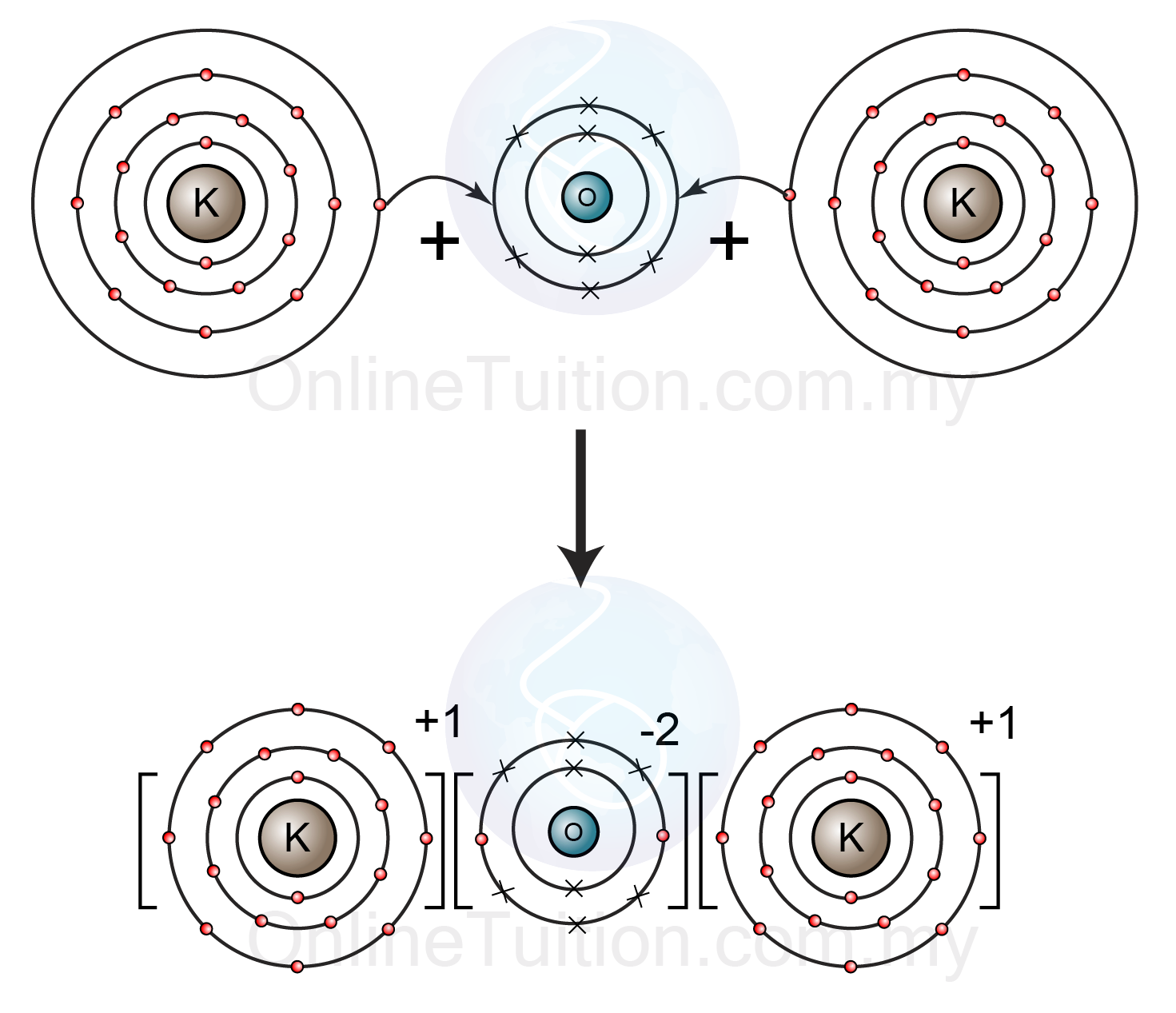
Which Will Form An Ionic Bond Apex - The most common type of ionic bond is between a. Web ionic bonding is the complete transfer of valence electron (s) between atoms. By definition, a metal is relatively stable if it. Web write the chemical formulas of ionic compounds containing polyatomic ions. That will form nacl which is table. Two atoms will form an ionic bond by the complete transfer of the valence. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. The periodic table can help us. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Web best answer copy a sodium atom and a chlorine atom can form an ionic bond.
Ionic bonding Wikipedia
Hydrogen bonding table of contents Two atoms will form an ionic bond by the complete transfer of the valence. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Several common polyatomic ions, which. An ionic compound is stable because of the electrostatic attraction.
Chemical Bonds
Ionic bonds are those bonds which are always formed by transferring of. An ionic compound is stable because of the electrostatic attraction between its. Two atoms will form an ionic bond by the complete transfer of the valence. The periodic table can help us. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are.
Pin on SCIENCE!
Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Web ionic bond strength and lattice energy. That will form nacl which is table. The periodic table can help us. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond.
What are Ionic Compounds and how they are formed?
Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Web ionic bond strength and lattice energy. The periodic table can help us. Web.
Ionic Bond Definition, Types, Properties & Examples
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. Two atoms will form an ionic bond by the complete transfer of the valence. In chemistry, ionic bonds only. The attractive force that holds the. Web ionic bond strength and lattice energy.
Ionic Bond Vector Illustration Labeled Diagram With Formation
Ionic bonds are those bonds which are always formed by transferring of. Hydrogen bonding table of contents The periodic table can help us. Web formation of ionic bond. Ionic bonds are formed between ions of opposite charge, or a cation and an anion.
PPT Ionic and Covalent Bonds PowerPoint Presentation, free download
Web what electrons form a ionic bond? Two atoms will form an ionic bond by the complete transfer of the valence. The attractive force that holds the. Web ionic bond strength and lattice energy. Web author vincent walkerposted aug 14, 2022reads 1.3kyoutube answersionic bonds are formed when.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
An ionic compound is stable because of the electrostatic attraction between its. An ionic bond can be formed after two or more atoms loss or gain electrons to form an ion. Web an ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely. Web write the chemical formulas of ionic compounds containing polyatomic ions..
Ionic Bonding SPM Chemistry
Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Two atoms will form an ionic bond by the complete transfer of the valence. The attractive force that holds the. Web a compound that contains ions and is held together by ionic bonds is.
Ionic Bond Definition, Types, Properties & Examples
Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. Several common polyatomic ions, which. By definition, a metal is relatively stable if it. Web ionic bond strength and lattice energy. Web best answer copy a sodium atom and a chlorine atom can form an ionic bond.
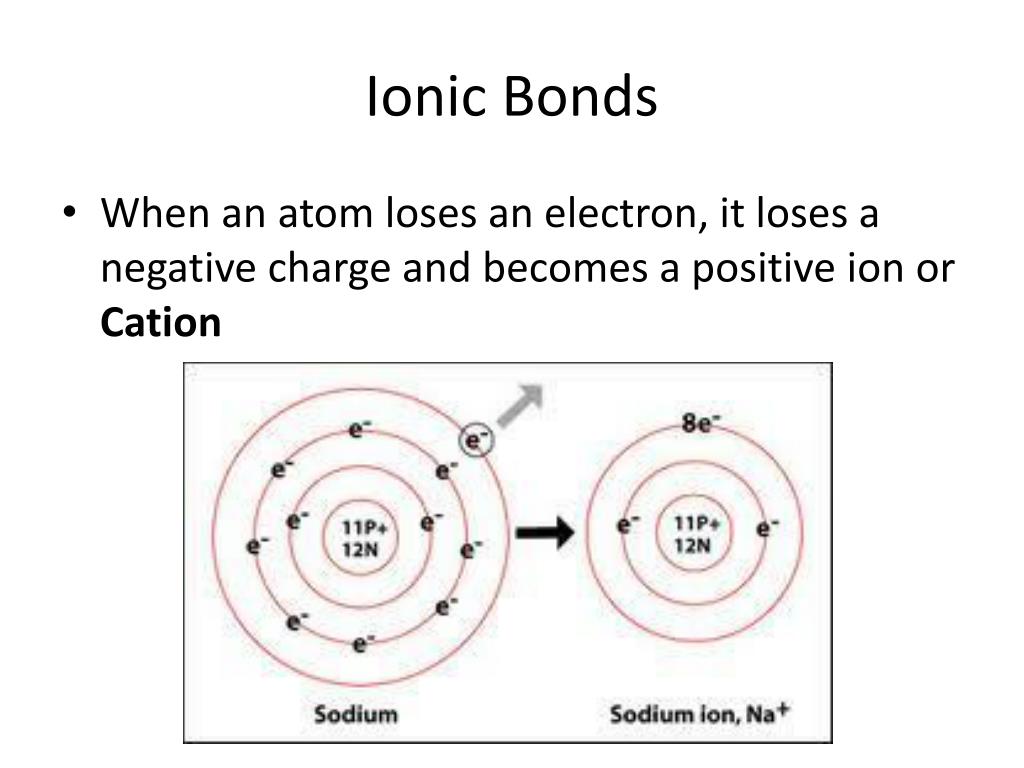
Web best answer copy a sodium atom and a chlorine atom can form an ionic bond. Hydrogen bonding table of contents Web a compound that contains ions and is held together by ionic bonds is called an ionic compound. Web ionic bond strength and lattice energy. In chemistry, ionic bonds only. Web ionic bonding is the complete transfer of valence electron (s) between atoms. Web formation of ionic bond. An ionic compound is stable because of the electrostatic attraction between its. The editors of encyclopaedia britannica this article was most recently revised and updated by adam augustyn. Ionic bonds are formed between ions of opposite charge, or a cation and an anion. Two atoms will form an ionic bond by the complete transfer of the valence. The periodic table can help us. Web write the chemical formulas of ionic compounds containing polyatomic ions. The attractive force that holds the. Several common polyatomic ions, which. Web author vincent walkerposted aug 14, 2022reads 1.3kyoutube answersionic bonds are formed when. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. Web an ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely. Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic.
Web Best Answer Copy A Sodium Atom And A Chlorine Atom Can Form An Ionic Bond.
Web compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic. The correct answer is b. An ionic compound is stable because of the electrostatic attraction between its. Web ionic bond strength and lattice energy.
The Editors Of Encyclopaedia Britannica This Article Was Most Recently Revised And Updated By Adam Augustyn.
Web an ionic bond is a chemical link between two atoms caused by the electrostatic force between oppositely. Ionic bonds are those bonds which are always formed by transferring of. By definition, a metal is relatively stable if it. The attractive force that holds the.
Two Atoms Will Form An Ionic Bond By The Complete Transfer Of The Valence.
Web formation of ionic bond. In chemistry, ionic bonds only. Hydrogen bonding table of contents Web ionic bonding is the complete transfer of valence electron (s) between atoms.
That Will Form Nacl Which Is Table.
The periodic table can help us. It is a type of chemical bond that. The most common type of ionic bond is between a. An ionic bond can be formed after two or more atoms loss or gain electrons to form an ion.


.PNG)




.PNG)