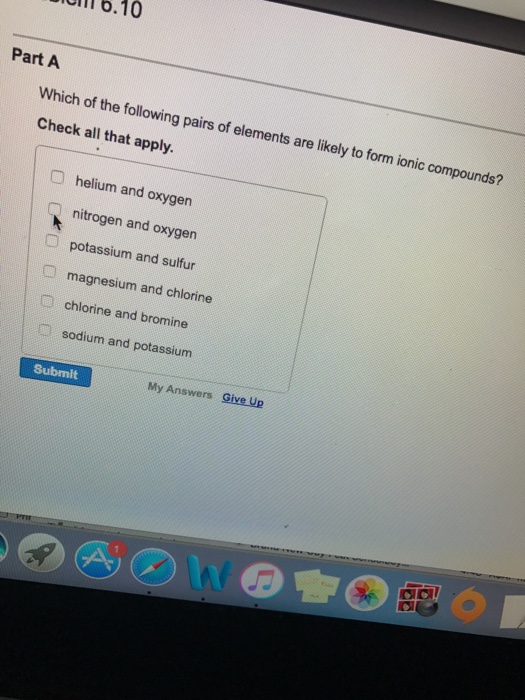
Which Pairs Of Elements Are Likely To Form Ionic Compounds - Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web which of the following pairs of elements would be most likely to form an ionic compound? Web magnesium and nitrogen react to form an ionic compound. The most common example of. Web a pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. Chlorine and bromine helium and oxygen. Web the strong electrostatic attraction between adjacent cations and anions is known as an ionic bond. These types of ionic compounds are. A iodine and bromine b hydrogen and iodine c potassium and.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Which of the following pairs of elements are likely to form ionic compounds? Web determine whether the following pairs of elements can form ionic compounds. Part a which of the following pairs of elements are likely to form an ionic.
Solved Which Of The Following Pairs Of Elements Are Likel...
The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and. Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Therefore, one lithium (li) cation. Web part a.
Examples of Ionic Bonding YouTube
Web although it is impossible to draw an exact line of demarcation, a good working rule is that essentially all binary. A iodine and bromine b hydrogen and iodine c potassium and. Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Web the pair of elements most likely to.
Chem Forming Ionic Compounds Scientific Tutor
Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and. A iodine and bromine b hydrogen and iodine c potassium and. Therefore, one lithium (li) cation. Web an expression that indicates the number and type of atoms present.
Ionic Bond Definition Easy Hard Science
Web a pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. Part a which of the following pairs of elements are likely to form an ionic compound? The most common example of. Web an expression that indicates the number and type of atoms present in the smallest representative unit.
Periodic Table Of Elements Names And Symbols List In Order Pdf
A iodine and bromine b hydrogen and iodine c potassium and. Compounds between metal and nonmetal elements are usually ionic. Web although it is impossible to draw an exact line of demarcation, a good working rule is that essentially all binary. Part a which of the following pairs of elements are likely to form an ionic compound? Web chemistry questions.
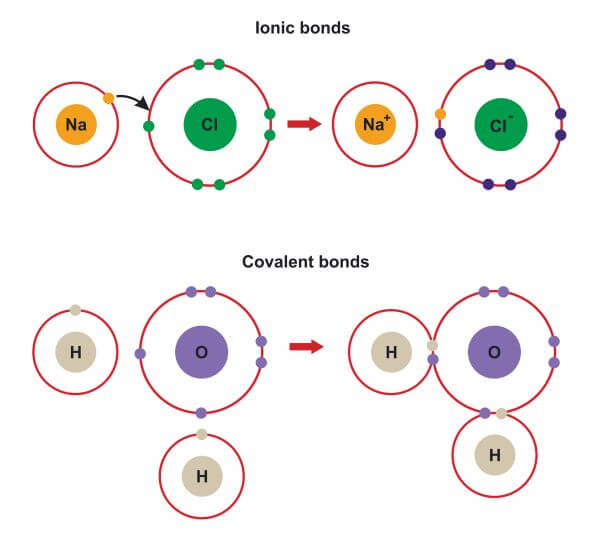
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
Web the strong electrostatic attraction between adjacent cations and anions is known as an ionic bond. Web chemistry questions and answers. Compounds between metal and nonmetal elements are usually ionic. Web determine whether the following pairs of elements can form ionic compounds. Web although it is impossible to draw an exact line of demarcation, a good working rule is that.
Ionic Bond Examples Biology Dictionary
Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Web which of the following pairs of elements would be most likely to form an ionic compound? Which of the following pairs of elements are likely to form ionic compounds? These types of ionic compounds are. A iodine and bromine.
Solved Which of the following pairs of elements are likely
Web which of the following pairs of elements would be most likely to form an ionic compound? Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; Therefore, the resultant ion is. Which of the following pairs of elements are likely to form ionic compounds? Compounds between metal and nonmetal elements are usually ionic.
⚗️Which of the following pairs of elements is most likely to form an
Chlorine and bromine helium and oxygen. Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; The correct pairs of elements likely to form ionic compounds are, potassium and sulfur, magnesium and. These types of ionic compounds are. Therefore, the resultant ion is.
Compounds between metal and nonmetal elements are usually ionic. A iodine and bromine b hydrogen and iodine c potassium and. Web to form ionic bonds, carbon molecules must either gain or lose 4 electrons. Web determine whether the following pairs of elements can form ionic compounds. Which of the following pairs of elements are likely to form ionic compounds? Web chemistry questions and answers. Web the strong electrostatic attraction between adjacent cations and anions is known as an ionic bond. Web which of the following pairs of elements would be most likely to form an ionic compound? Web the pair of elements most likely to form an ionic compound are : Part a which of the following pairs of elements are likely to form an ionic compound? Chlorine and bromine helium and oxygen. Web although it is impossible to draw an exact line of demarcation, a good working rule is that essentially all binary. Web a pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal. Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance. Web part a which of the following pairs of elements are likely to form an ionic compound? Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; Which of the following pairs of elements are likely to form ionic compounds? Web magnesium and nitrogen react to form an ionic compound. The most common example of.
Therefore, One Lithium (Li) Cation.
Occur when the valence electrons of atoms of a metal are transferred to atoms of nonmetals; Which of the following pairs of elements are likely to form ionic compounds? A iodine and bromine b hydrogen and iodine c potassium and. Web a pair of elements will most likely form an ionic bond if one is a metal and one is a nonmetal.
Web Determine Whether The Following Pairs Of Elements Can Form Ionic Compounds.
Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. Chlorine and bromine helium and oxygen. Compounds between metal and nonmetal elements are usually ionic. Web chemistry questions and answers.
Web To Form Ionic Bonds, Carbon Molecules Must Either Gain Or Lose 4 Electrons.
Web magnesium and nitrogen react to form an ionic compound. These types of ionic compounds are. Web the strong electrostatic attraction between adjacent cations and anions is known as an ionic bond. Web an expression that indicates the number and type of atoms present in the smallest representative unit of a substance.
Part A Which Of The Following Pairs Of Elements Are Likely To Form An Ionic Compound?
The most common example of. Web part a which of the following pairs of elements are likely to form an ionic compound? Therefore, the resultant ion is. Which of the following pairs of elements are likely to form ionic compounds?

.PNG)