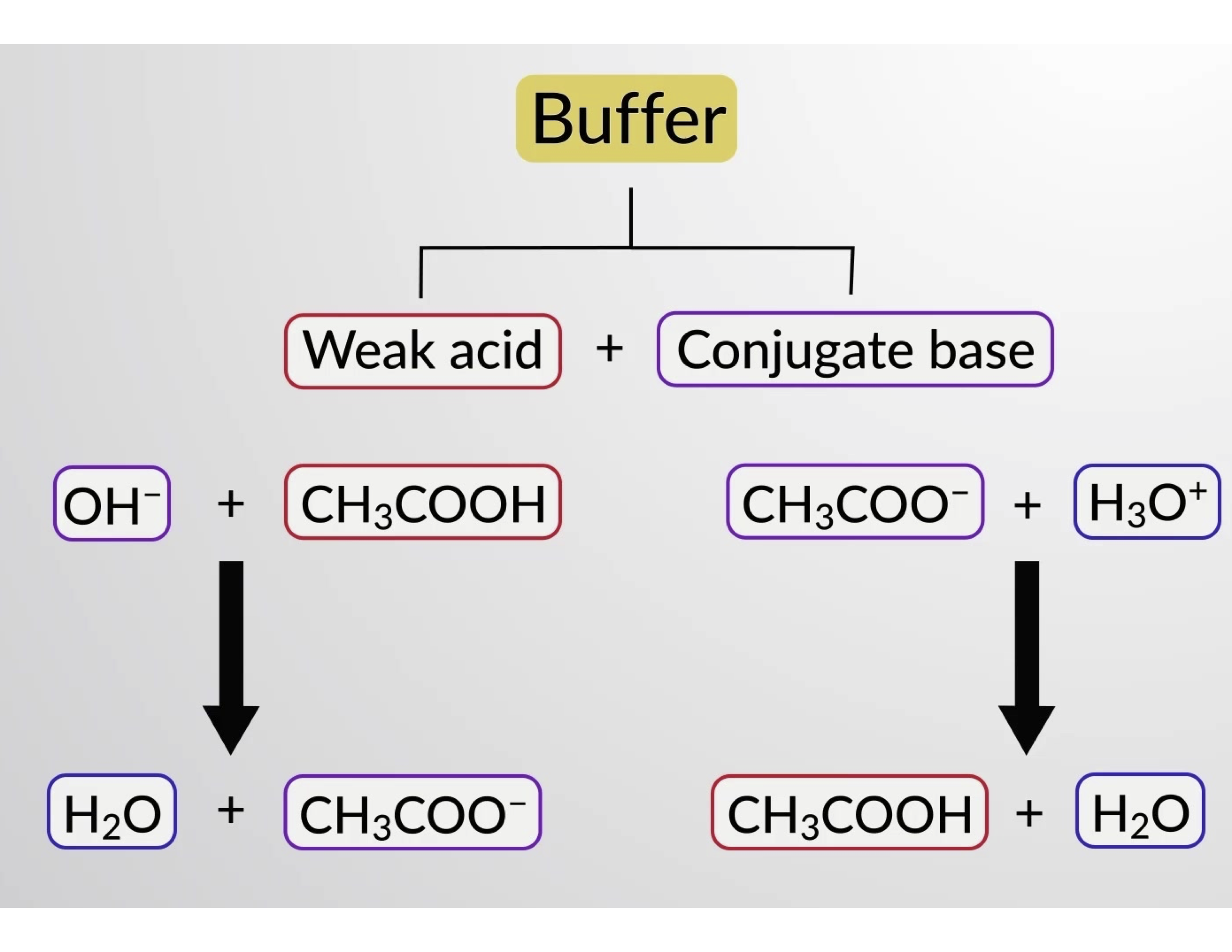
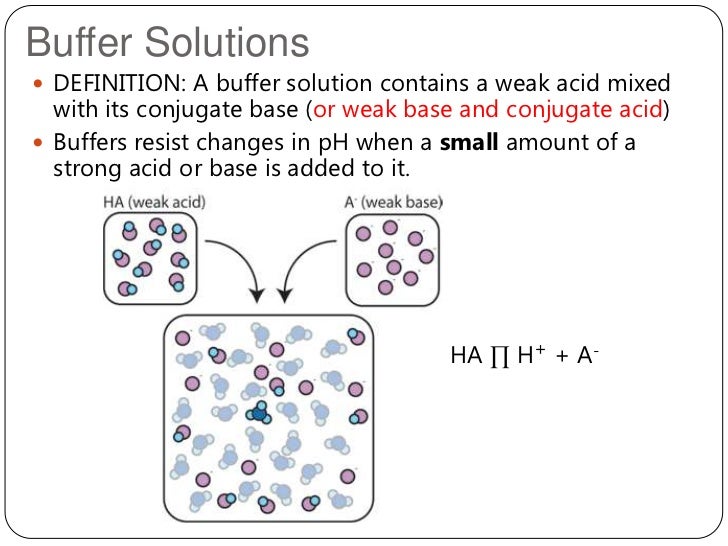
Which Pair Of Solutions Will Form An Effective Buffer - Web a solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Web the pair (s) of solutions which form a buffer upon mixing is(are)? Web which pair of solutions will form an effective buffer? Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together. Web let’s go to a chemistry lab and conduct a simple experiment. Either a weak acid plus its conjugate base or a. The ph of solution a. Web which pair of solutions will form an effective buffer? In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an. Either a weak acid plus a salt derived from that weak acid or a weak base.
18.2.1 Describe the composition of a buffer solution and explain its
Most of the important buffer solutions generally consist of mixture of weak acids and their salts or weak. Web which pair of solutions will form an effective buffer? The ph of solution a. Web an effective buffer is a solution that can resist significant changes in ph when small amounts of an acid or base. Web let’s go to a.
Which Pair Will Produce a Buffer Solution AllysonhasMccoy
Web correct option is a) 1. Web the pair (s) of solutions which form a buffer upon mixing is(are)? Either a weak acid plus a salt derived from that weak acid or a weak base. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn. 1.0 m hcn and 0.30 m kcn what.
Buffers Presentation Chemistry
0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn. Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together. Web types of buffer solution. Either a weak acid plus its conjugate base or a. Web which pair of aqueous solutions can create.
Buffers, Buffer Components and Buffer Action Chemistry JoVE
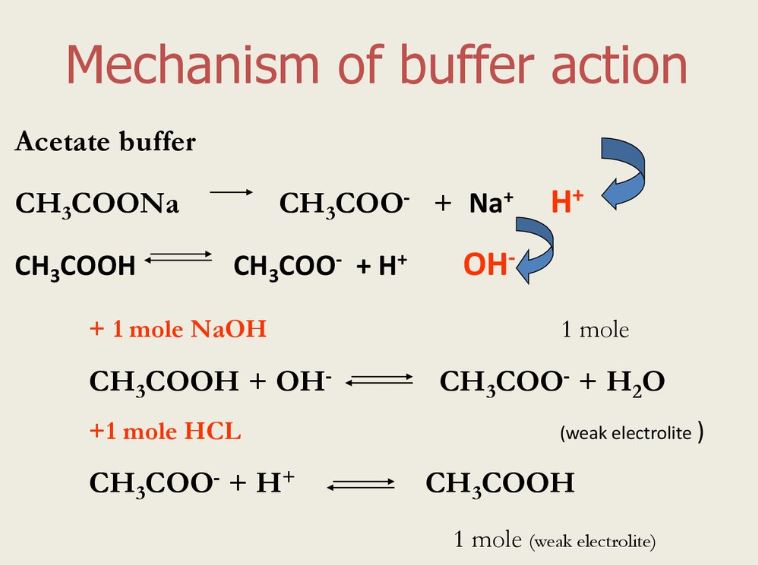
There are two buffer forms, acid buffer, and base buffer. Either a weak acid plus its conjugate base or a. The correct answer 0.40 m hf and 0.30 m naf a buffer is effective when the. Web which pair of solutions will form an effective buffer? As shown below, we have.
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007
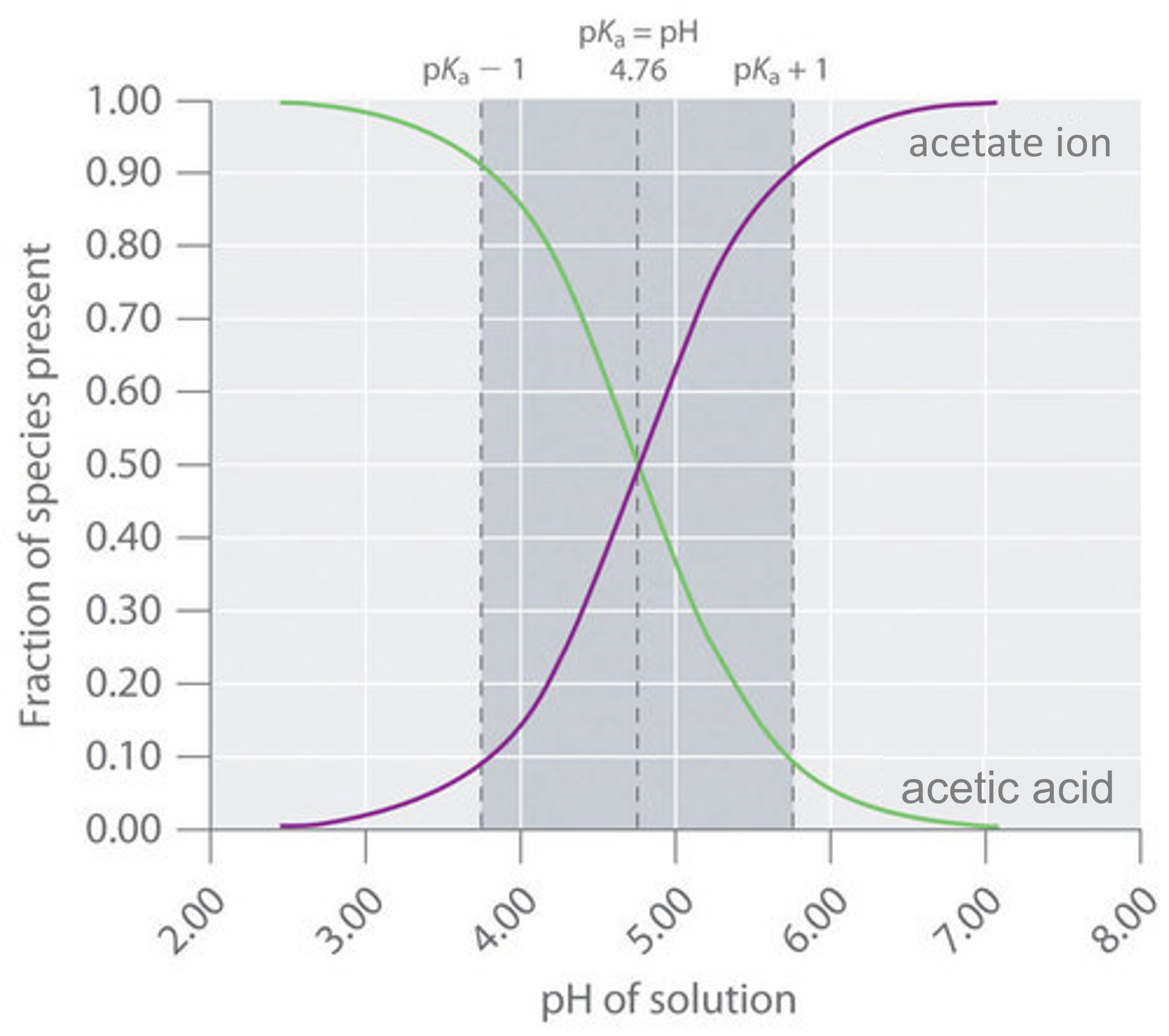
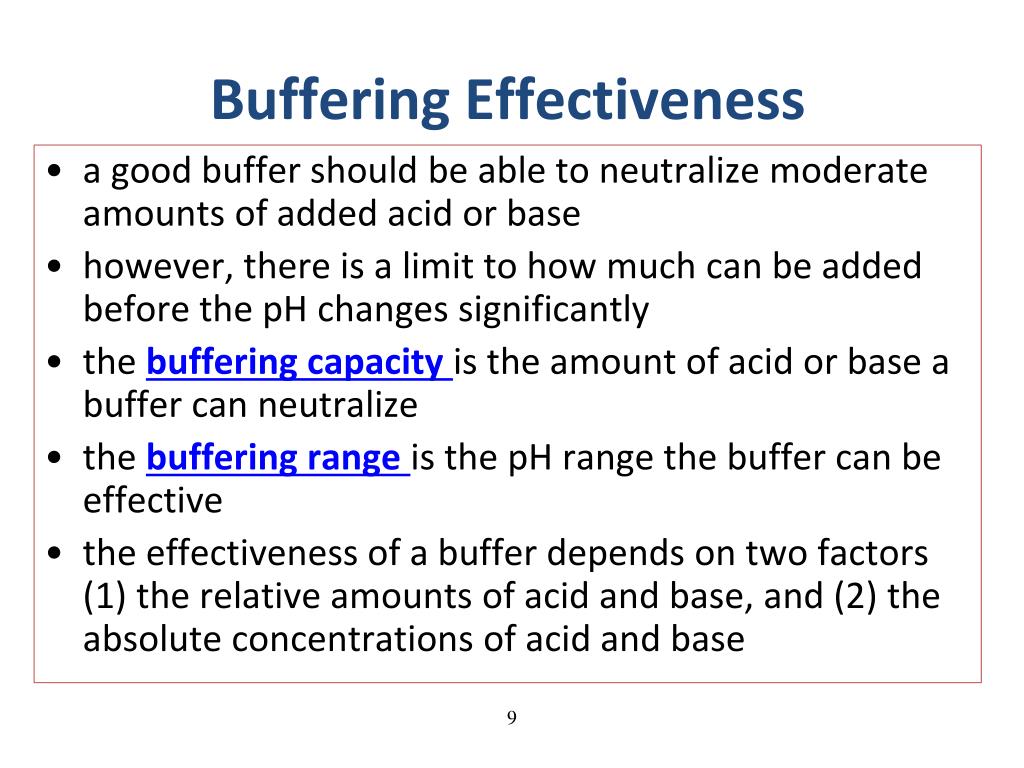
Web an effective buffer is a solution that can resist significant changes in ph when small amounts of an acid or base. Web a buffer's capacity is the ph range where it works as an effective buffer, preventing large changes in ph upon addition of an acid or. There are mainly two types of buffer solutions: Web a buffer solution.
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium
Web which pair of solutions will form an effective buffer? The correct answer 0.40 m hf and 0.30 m naf a buffer is effective when the. Web buffers do so by being composed of certain pairs of solutes: Web buffers do so by being composed of certain pairs of solutes: Web a buffer solution is formed when appreciable quantities of.
8.8 Buffers Solutions That Resist pH Change Chemistry LibreTexts
Buffers do so by being. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn. 1.0 m hcn and 0.30 m kcn what is the ph of a buffer system that has 0.11 m. Web correct option is a) 1. Either a weak acid plus a salt derived from that weak acid or.
PPT Chapter 16 Aqueous Ionic Equilibrium PowerPoint Presentation
Web let’s go to a chemistry lab and conduct a simple experiment. Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together. Web an effective buffer is a solution that can resist significant changes in ph when small amounts of an acid or base. Web types of buffer solution. Web.
PPT Chapter 16 Introduction to Buffers PowerPoint Presentation, free
Web which pair of solutions will form an effective buffer? Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together. Web a buffer that contains approximately equal amounts of a weak acid and its conjugate base in solution is equally effective at. In chemistry, the definition of a buffer is.
2012 topic 18 2 buffer solutions
Web which pair of aqueous solutions can create a buffer solution if present in the appropriate concentrations? Web what are the types of buffer solutions? Web which pair of solutions will form an effective buffer? Web which pair of solutions will form an effective buffer? Web a buffer that contains approximately equal amounts of a weak acid and its conjugate.
0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn. Web which pair of solutions will form an effective buffer? Web a solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Web buffers do so by being composed of certain pairs of solutes: Web an effective buffer is a solution that can resist significant changes in ph when small amounts of an acid or base. Web which pair of solutions will form an effective buffer? Web correct option is a) 1. 1.0 m hcn and 0.30 m kcn what is the ph of a buffer system that has 0.11 m. The ph of solution a. Web buffers do so by being composed of certain pairs of solutes: Web this mechanism involves a buffer, a solution that resists dramatic changes in ph. Buffers do so by being. Either a weak acid plus a salt derived from that weak acid or a weak. The correct answer 0.40 m hf and 0.30 m naf a buffer is effective when the. There are mainly two types of buffer solutions: In chemistry, the definition of a buffer is a solution that can resist ph change upon the addition of an. Web let’s go to a chemistry lab and conduct a simple experiment. Web the pair (s) of solutions which form a buffer upon mixing is(are)? Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together. Web which pair of aqueous solutions can create a buffer solution if present in the appropriate concentrations?
Web An Effective Buffer Is A Solution That Can Resist Significant Changes In Ph When Small Amounts Of An Acid Or Base.
Web a solution containing a mixture of an acid and its conjugate base, or of a base and its conjugate acid, is called a buffer solution. Either a weak acid plus a salt derived from that weak acid or a weak. 0.35 m hf and 0.45 m hno2 0.50 m nh3 and 0.50 m ch3cooh 1.0 m hcn. Web what are the types of buffer solutions?
Web A Buffer That Contains Approximately Equal Amounts Of A Weak Acid And Its Conjugate Base In Solution Is Equally Effective At.
Web correct option is a) 1. Web this mechanism involves a buffer, a solution that resists dramatic changes in ph. The ph of solution a. Web let’s go to a chemistry lab and conduct a simple experiment.
In Chemistry, The Definition Of A Buffer Is A Solution That Can Resist Ph Change Upon The Addition Of An.
Web buffers do so by being composed of certain pairs of solutes: Buffers do so by being. Web a buffer's capacity is the ph range where it works as an effective buffer, preventing large changes in ph upon addition of an acid or. Web a buffer solution is formed when appreciable quantities of a weak acid and its conjugate base are mixed together.
Most Of The Important Buffer Solutions Generally Consist Of Mixture Of Weak Acids And Their Salts Or Weak.
Either a weak acid plus its conjugate base or a. Either a weak acid plus a salt derived from that weak acid or a weak base. There are two buffer forms, acid buffer, and base buffer. Web which pair of solutions will form an effective buffer?


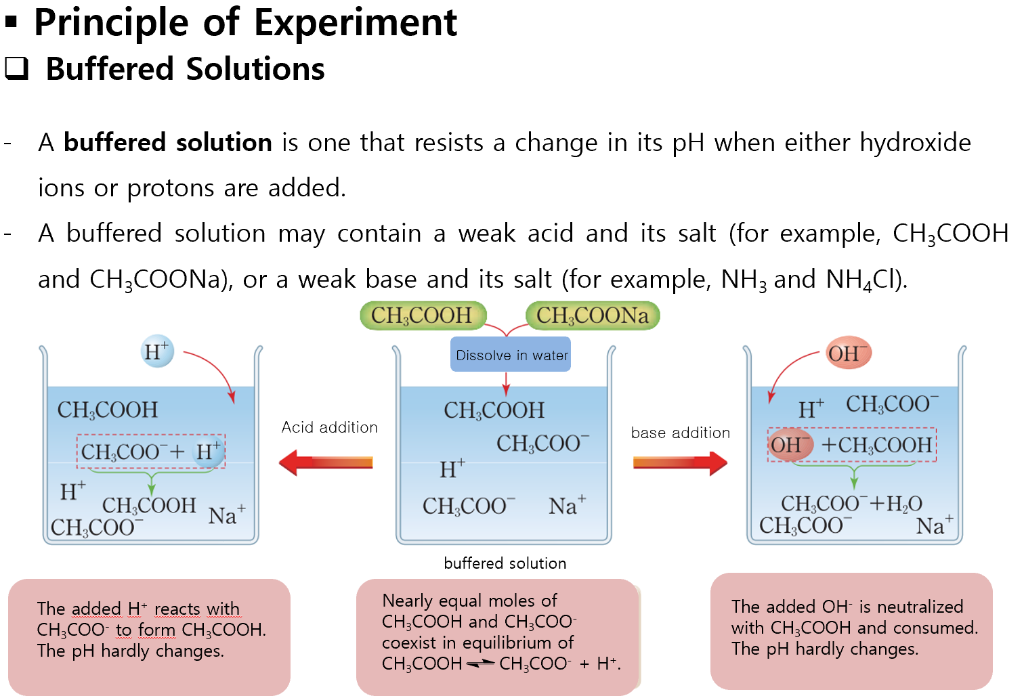
.PNG)