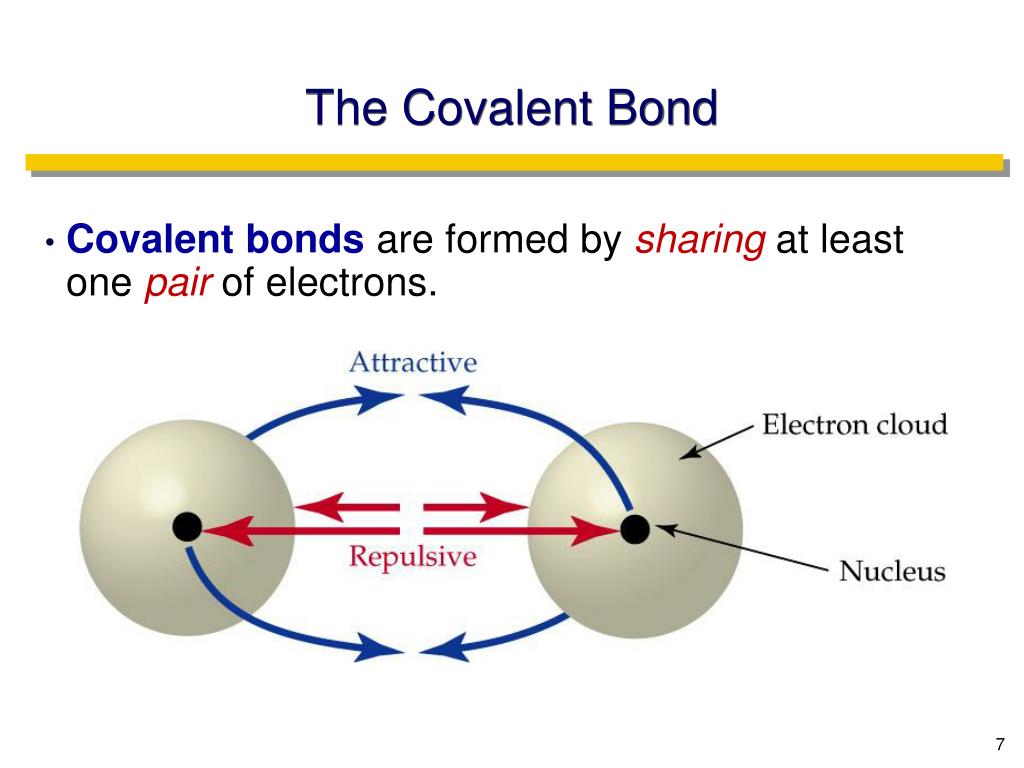
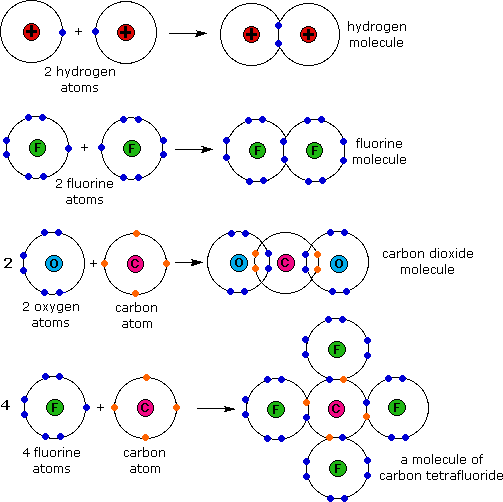
Which Pair Of Elements Will Form A Covalent Bond - Web a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence electrons by sharing two. Web bonds between two nonmetals are generally covalent; Electron pairs shared between atoms of equal or very. The two element pair that will form covalent bonds in a compound are i and f. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Bonding between a metal and a nonmetal is often ionic. It is this sharing of electrons that we refer. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. In a covalent bond, the stability of the bond comes from the. Web covalent bonds involve the sharing of electron pairs between atoms.
Examples of Covalent Bonds Several Examples of Covalent (molecular
This type of bonding occurs. In a covalent bond, the stability of the bond comes from the. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Covalent bonding is the sharing of electrons between atoms. Web bonds between two nonmetals are generally covalent;
Which elements form a covalent bond? Quora
Web each atom donates a single electron to the electron pair which is shared. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Bonding between a metal and a nonmetal is often ionic. Web a covalent bond is a chemical bond that involves the.
The top panel in this figure shows two hydrogen atoms sharing two
Covalent bonding is the sharing of electrons between atoms. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web bonds between two nonmetals are generally covalent; If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules,. Electron pairs shared.
Covalent Bonding (Biology) — Definition & Role Expii
Bonding between a metal and a nonmetal is often ionic. Electronegativities are used to determine. Web a pair of electrons that is shared between two atoms is called a bond pair. Electron pairs shared between atoms of equal or very. Web bonds between two nonmetals are generally covalent;
chemistry picture
Web a covalent bond is formed when two atoms share electron pairs. Covalent bonding is the sharing of electrons between atoms. Web covalent bonds are formed by the sharing of electrons.generally organic compound are formed as the reason of. The two element pair that will form covalent bonds in a compound are i and f. Web the sharing of electrons.
PPT Covalent Bonding PowerPoint Presentation, free download ID4003633
Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Electronegativities are used to determine. Web bonds between two nonmetals are generally covalent; The two element pair that will form covalent bonds in a compound are i and f. Web covalent bonds involve the sharing.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. It is this sharing of electrons that we refer. Covalent bonding is the sharing of electrons between atoms. Web atoms that share pairs of electrons form molecules. Web each atom donates a single electron to the electron pair which is.
EduMission Chemistry Form 4 Chapter 5 Covalent Bond
Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. The two element pair that will form covalent bonds in a compound are i and f. Web each atom donates a single electron to the electron pair which is shared. Electron pairs shared between atoms.
CH150 Chapter 4 Covalent Bonds and Molecular Compounds Chemistry
Web one way to predict the type of bond that forms between two elements is to consider whether each element is a metal or. Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively. Web a pair of electrons that is shared between two atoms is called a bond.
Covalent Bond Biology Dictionary
A pair of electrons that is not shared between two atoms is called a lone pair. Electron pairs shared between atoms of equal or very. Web each atom donates a single electron to the electron pair which is shared. Electronegativities are used to determine. Web atoms that share pairs of electrons form molecules.
It is this sharing of electrons that we refer. Web which pair of atoms will form a covalent bond? Web a covalent bond is formed when two atoms share electron pairs. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence electrons by sharing two. This type of bonding occurs. Web covalent bonds are formed by the sharing of electrons.generally organic compound are formed as the reason of. Bonding between a metal and a nonmetal is often ionic. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements. Web the sharing of electrons between atoms is called a covalent bond, and the two electrons that join atoms in a covalent bond are. The electrons involved are in the outer shells. Web one way to predict the type of bond that forms between two elements is to consider whether each element is a metal or. Web bonds between two nonmetals are generally covalent; Web each atom donates a single electron to the electron pair which is shared. Web a pair of electrons that is shared between two atoms is called a bond pair. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. Electron pairs shared between atoms of equal or very. A pair of electrons that is not shared between two atoms is called a lone pair. Web atoms that share pairs of electrons form molecules. The two element pair that will form covalent bonds in a compound are i and f.
A Molecule Is A Group Of Atoms Held Together By Covalent Bonds.
Electronegativities are used to determine. Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively. Web a covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. Web one way to predict the type of bond that forms between two elements is to compare the electronegativities of the elements.
The Two Element Pair That Will Form Covalent Bonds In A Compound Are I And F.
If the atoms that form a covalent bond are identical, as in h 2, cl 2, and other diatomic molecules,. Web the number of bonds that an atom can form can often be predicted from the number of electrons needed to reach an octet (eight. The electrons involved are in the outer shells. Bonding between a metal and a nonmetal is often ionic.
Web The Sharing Of Electrons Between Atoms Is Called A Covalent Bond, And The Two Electrons That Join Atoms In A Covalent Bond Are.
Covalent bonding is the sharing of electrons between atoms. Web covalent bonds are formed by the sharing of electrons.generally organic compound are formed as the reason of. A pair of electrons that is not shared between two atoms is called a lone pair. Electron pairs shared between atoms of equal or very.
Web One Way To Predict The Type Of Bond That Forms Between Two Elements Is To Consider Whether Each Element Is A Metal Or.
Web atoms that share pairs of electrons form molecules. Web a pair of electrons that is shared between two atoms is called a bond pair. Web which pair of atoms will form a covalent bond? Web a pair of oxygen atoms can form an o 2 molecule in which each atom has a total of eight valence electrons by sharing two.