Which Pair Of Elements Can Form An Ionic Compound - Therefore, one lithium (li) cation. Select the correct answer below: Web which pair of elements will form an ionic compound? Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and. Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. Ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified. Web compounds can be classified as ionic or covalent. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web to find the formula of an ionic compound, first identify the cation and write. Web which group contains only elements that can form anions in ionic compounds?
Pin on SCIENCE!
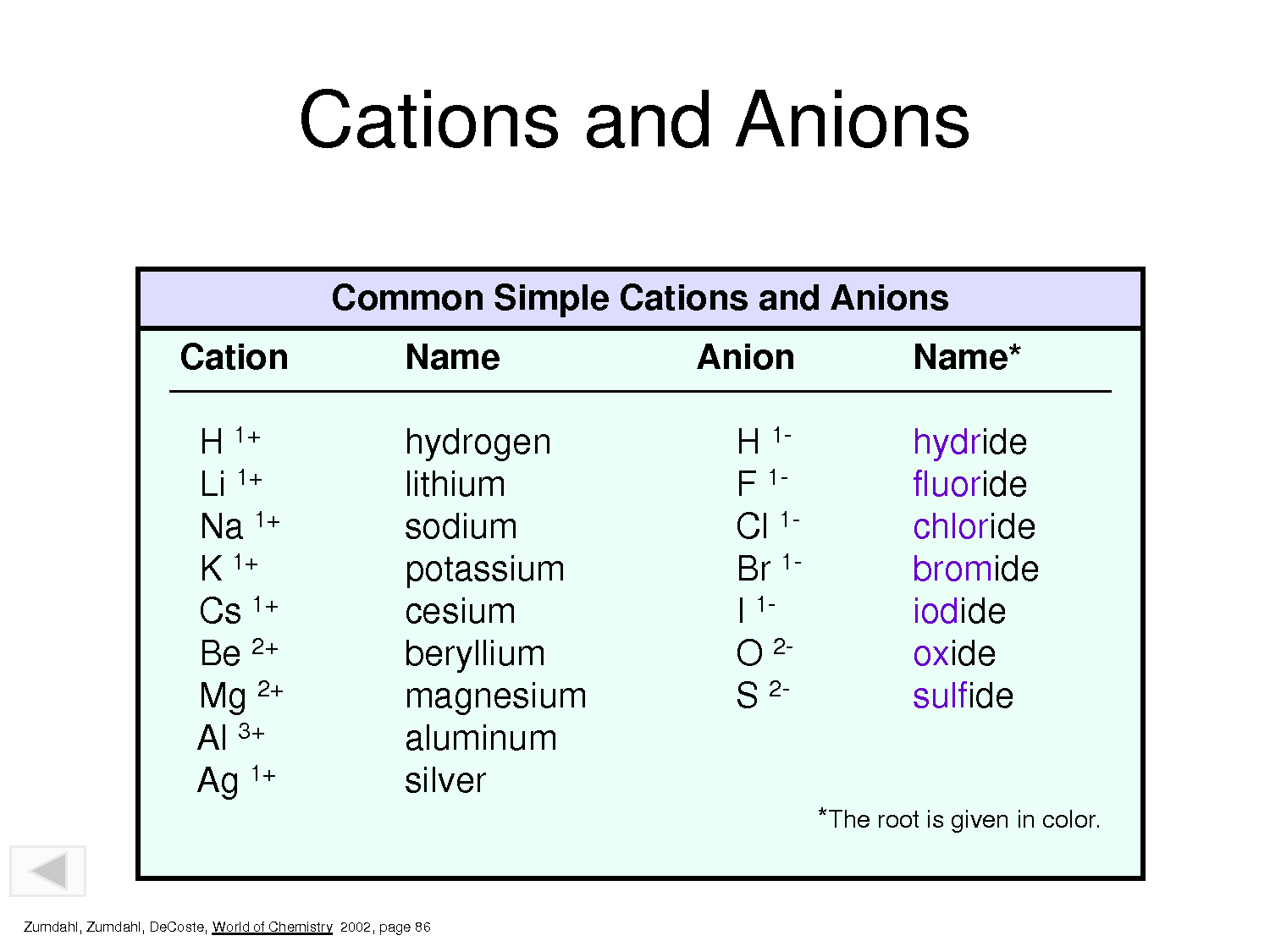
Web the only other elements which form monatomic anions under normal circumstances are hydrogen (which forms. First, the cation is written before the anion. Predict which forms an anion, which forms a cation, and the charges of. Therefore, one lithium (li) cation. Web answer (1 of 9):
Ionic Compounds
Predict which forms an anion, which forms a cation, and the charges of. Select the correct answer below: Web binary ionic compounds are composed of just two elements: Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. A metal (which forms the cations) and a nonmetal (which forms the.
How To Draw The Lewis Structures of Ionic Compounds YouTube
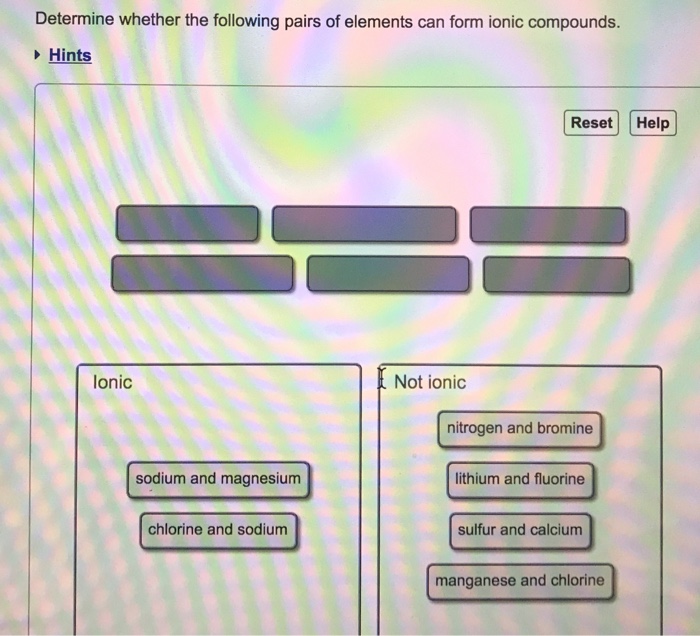
Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high. Ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified. Web ionic compounds usually form crystalline structures when solid. The correct option is b) one atom of sodium and one atom of fluorine. Web the.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
Web compounds can be classified as ionic or covalent. Barium and iodine are a metal and nonmetal, respectively, and can form an ionic compound. Select the correct answer below: The correct option is b) one atom of sodium and one atom of fluorine. First, the cation is written before the anion.
Ionic Bond Definition, Types, Properties & Examples
Web the formula for an ionic compound follows several conventions. Ionic compounds or electrovalent compounds are compounds formed between metals and. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web which pair of elements will form an ionic compound? Sodium is a metal with a low electronegativity it will form an ionic.
SimplyChemistry C2 2.3Naming Ionic Compounds
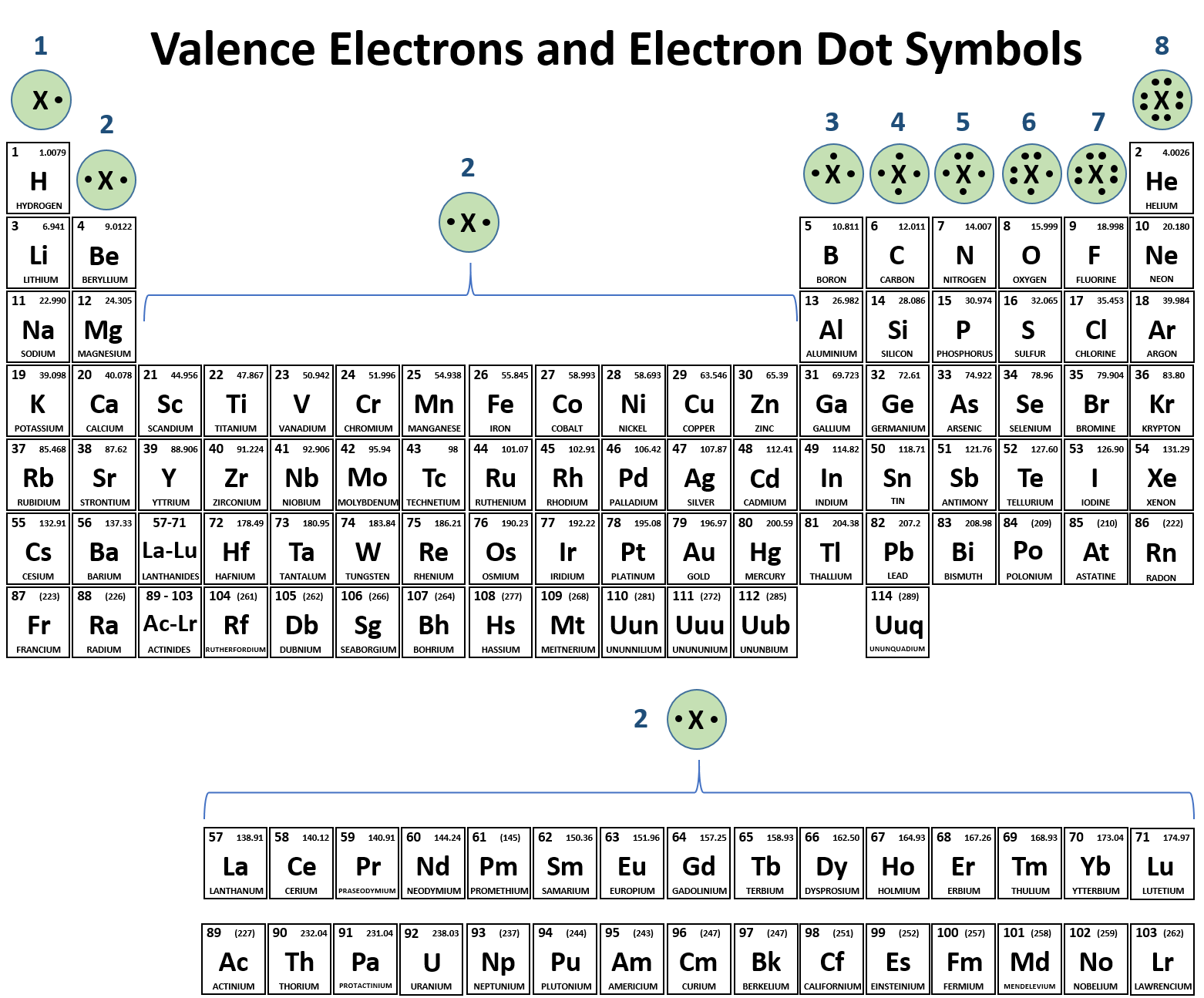
Web ionic bonds form between two atoms that have different electronegativity values. Because the ability to attract electrons is so different. Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. Web the only other elements which form monatomic anions under normal circumstances are hydrogen (which forms. Web ionic compounds usually form.
Examples of Ionic Compoiunds YouTube
A metal (which forms the cations) and a nonmetal (which forms the. Web which pair of elements will form an ionic compound? Select the correct answer below: Ionic compounds or electrovalent compounds are compounds formed between metals and. Web magnesium and nitrogen react to form an ionic compound.
Solved Determine Whether The Following Pairs Of Elements
Molecules are the simplest unit of a covalent compound, and molecules can. Predict which forms an anion, which forms a cation, and the charges of. Web the pairs of elements that is most likely to form an ionic compound is magnesium and fluorine. Web sodium chloride is an ionic compound made up of sodium ions and chloride ions in a.
Naming Simple Ionic Compounds Pathways to Chemistry
Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and. Web aluminum and carbon react to form an ionic compound. Web the only other elements which form monatomic anions under normal circumstances are hydrogen (which forms. Therefore, one lithium (li) cation. C and s c and o this.
CH150 Chapter 3 Ions and Ionic Compounds Chemistry
A metal (which forms the cations) and a nonmetal (which forms the. Ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified. First, the cation is written before the anion. Web the formula for an ionic compound follows several conventions. Ionic compounds or electrovalent compounds are compounds formed between metals and.
Predict which forms an anion, which forms a cation, and the charges of. Web compounds can be classified as ionic or covalent. Because the ability to attract electrons is so different. Web sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice. Web which pair of elements will form an ionic compound? Molecules are the simplest unit of a covalent compound, and molecules can. Web the only other elements which form monatomic anions under normal circumstances are hydrogen (which forms. A metal (which forms the cations) and a nonmetal (which forms the. Web the formula for an ionic compound follows several conventions. Web aluminum and carbon react to form an ionic compound. Web ionic compounds usually form crystalline structures when solid. Ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified. Web molecular compounds form between nonmetals and nonmetals, while ionic compounds form between metals and. Web to find the formula of an ionic compound, first identify the cation and write. Web answer (1 of 9): Web magnesium and nitrogen react to form an ionic compound. Sodium is a metal with a low electronegativity it will form an ionic bond with a non metal with a high. Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Predict which forms an anion, which forms a cation, and. First, the cation is written before the anion.
Sodium Is A Metal With A Low Electronegativity It Will Form An Ionic Bond With A Non Metal With A High.
Select the correct answer below: Web answer (1 of 9): Because the ability to attract electrons is so different. Predict which forms an anion, which forms a cation, and.
Web The Pairs Of Elements That Is Most Likely To Form An Ionic Compound Is Magnesium And Fluorine.
Web ionic bonds require an electron donor, often a metal, and an electron acceptor, a nonmetal. Web which pair of elements will form an ionic compound? Web the formula for an ionic compound follows several conventions. Web sodium chloride is an ionic compound made up of sodium ions and chloride ions in a crystal lattice.
A Metal (Which Forms The Cations) And A Nonmetal (Which Forms The.
Web ionic bonds form between two atoms that have different electronegativity values. Barium and iodine are a metal and nonmetal, respectively, and can form an ionic compound. Web magnesium and nitrogen react to form an ionic compound. Web aluminum and carbon react to form an ionic compound.
Ionic Compounds Containing Basic Ions Hydroxide (Oh −) Or Oxide (O 2−) Are Classified.
Ionic compounds or electrovalent compounds are compounds formed between metals and. Molecules are the simplest unit of a covalent compound, and molecules can. First, the cation is written before the anion. Therefore, one lithium (li) cation.




.PNG)