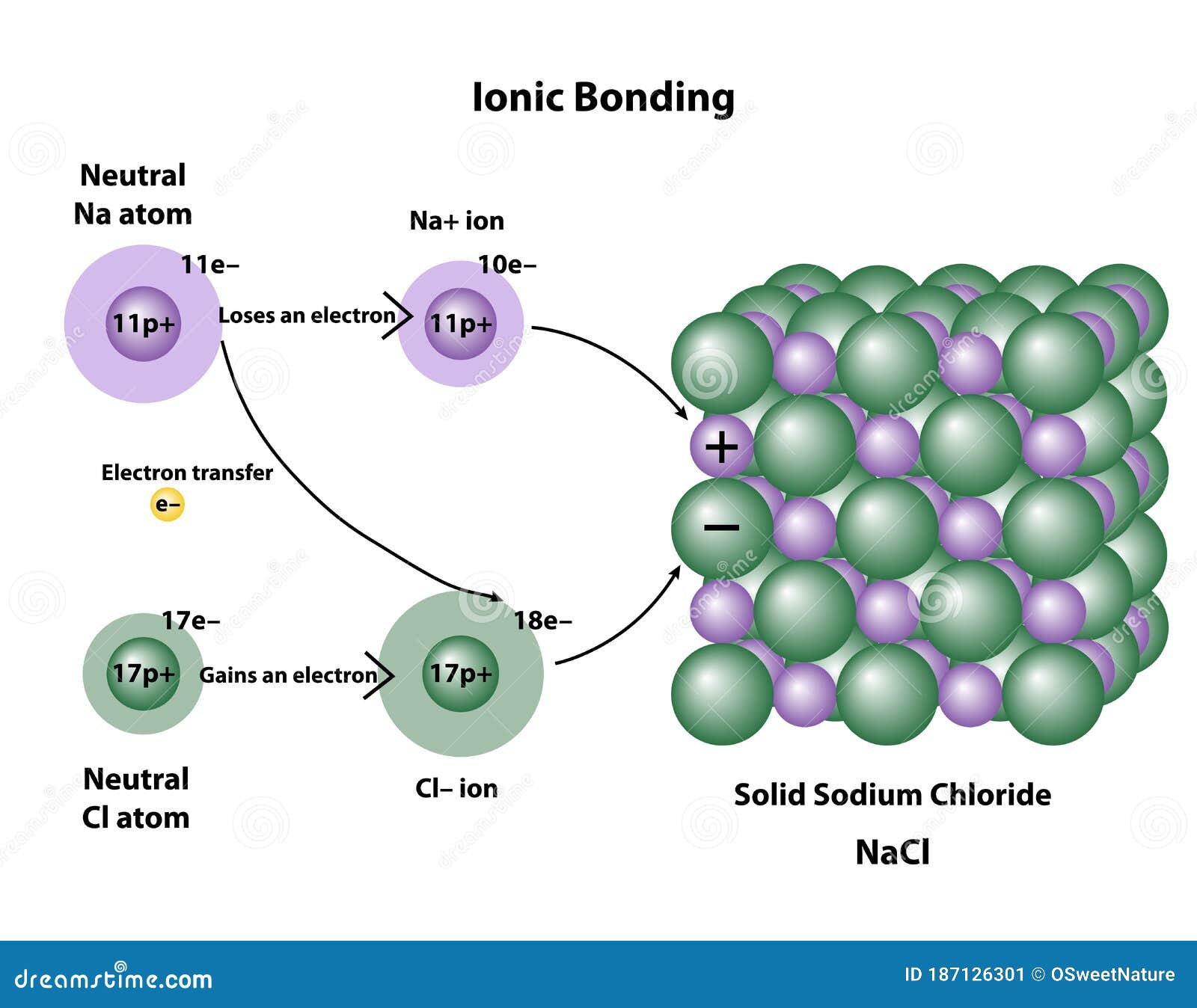
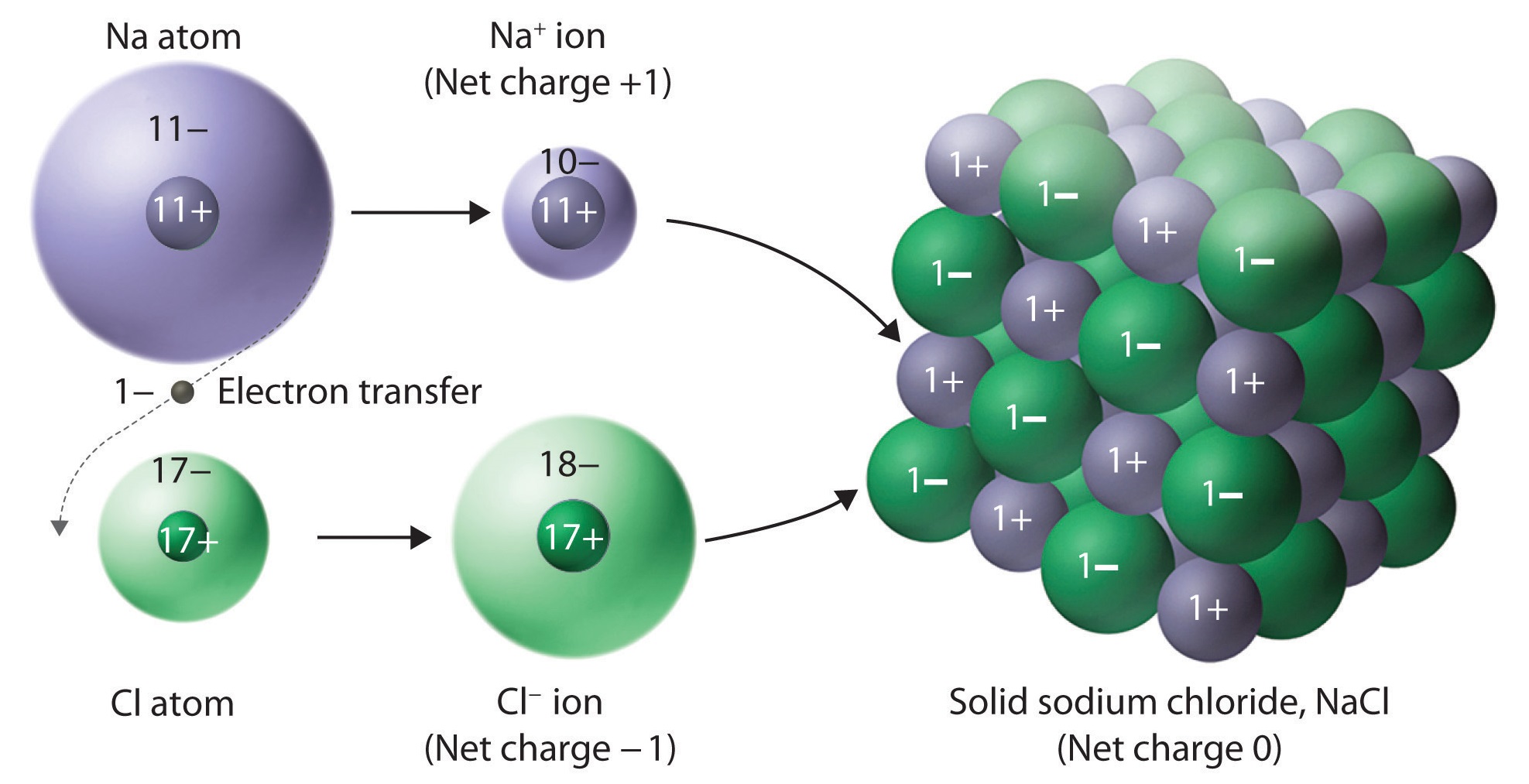
Which Of The Following Forms An Ionic Solid - 100% (2 ratings) rbi forms an ionic solid because it have both m. Web generally, ionic crystals form from a combination of group 1 or 2 metals and group 16 or 17 nonmetals or. Web aug 14, 2020 12.4: Crystal structures learning objectives to understand the correlation. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Ionic solids are made up of positive ion cation and negative ion anion. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. Net ionic equations show only the ions and. Alright, so remember, an ionic solid is an ionic compound. Rbi is called as rubidium iodide, which is made up of rubidium cation and.
IGCSE Chemistry 2017 1.56C Understand Why Ionic Compounds Conduct
Most ionic solids and molecular solids are insulators because of the negligible concentration of conduction. The ionic compound nacl forms when electrons from sodium atoms are transferred to chlorine atoms. Rbi is called as rubidium iodide, which is made up of rubidium cation and. Web this problem has been solved! Web chemistry chemistry questions and answers which of the following.
Network Atomic Solids
N2h4 c3h7nh2 co cuso4 this problem. The lattice energy δhlattice of. Web chemistry chemistry questions and answers which of the following forms an ionic solid? Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Web this problem has been solved!
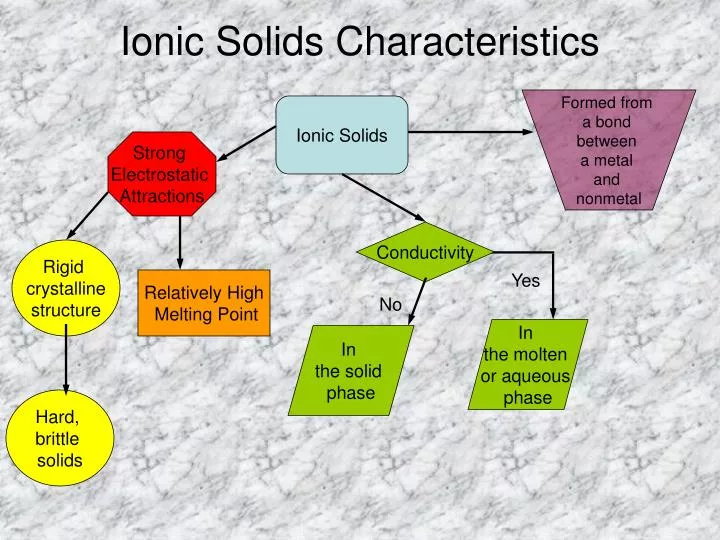
PPT Ionic Solids Characteristics PowerPoint Presentation, free
100% (2 ratings) rbi forms an ionic solid because it have both m. Web ionic compounds usually form crystalline structures when solid. Web ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Alright, so remember, an ionic solid is an ionic compound. For example, the sodium ions attract chloride ions and the chloride.
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation
Web ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures. Web complete ionic equations show dissolved ionic solids as separated ions. Web chemistry chemistry questions and answers which of the following forms an ionic solid? Web ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Web this problem has.
What are Ionic Compounds and how they are formed?
In nature, the ordered arrangement of ionic solids gives. Rbi is called as rubidium iodide, which is made up of rubidium cation and. Web chemistry chemistry questions and answers which of the following forms an ionic solid? Web aug 14, 2020 12.4: Web complete ionic equations show dissolved ionic solids as separated ions.
What Is An Ionic Compound? Formula and Defination
Web ionic compounds usually form crystalline structures when solid. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. 100% (2 ratings) rbi forms an ionic solid because it have both m. Crystal structures learning objectives to understand.
Ionic Bonding Presentation Chemistry
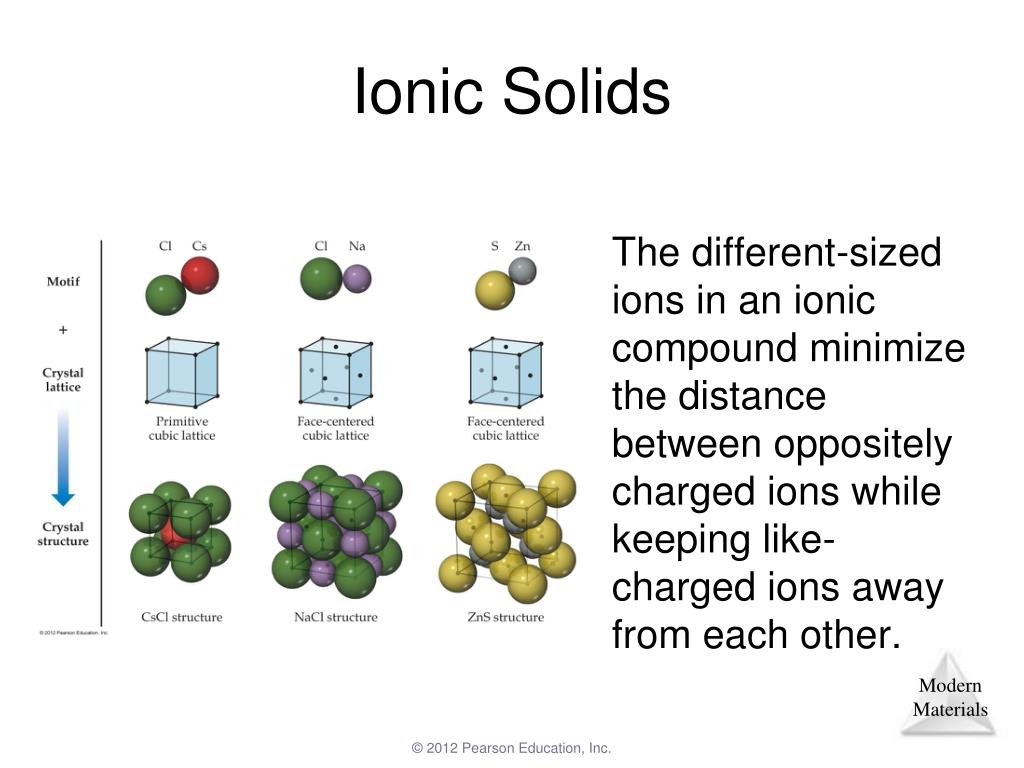
The lattice energy δhlattice of. Crystal structures learning objectives to understand the correlation. Which of the substances would form an ionic solid? In nature, the ordered arrangement of ionic solids gives. Ionic solids are made up of positive ion cation and negative ion anion.
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation
Web this problem has been solved! The lattice energy δhlattice of. N2h4 c3h7nh2 co cuso4 this problem. Rbi is called as rubidium iodide, which is made up of rubidium cation and. Crystal structures learning objectives to understand the correlation.
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector
Web this problem has been solved! Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. The ionic compound nacl forms when electrons from sodium atoms are transferred to chlorine atoms. Ionic solids are made up of positive ion cation and negative ion anion. Web chemistry chemistry questions and answers which of the.
Ionic Solids Chemistry LibreTexts
Web ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. In nature, the ordered arrangement of ionic solids gives. Web identify the ionic solid from the following options. The lattice energy δhlattice of. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions.
The lattice energy δhlattice of. Ionic solids are made up of positive ion cation and negative ion anion. 100% (2 ratings) rbi forms an ionic solid because it have both m. Web complete ionic equations show dissolved ionic solids as separated ions. Ionic compounds containing basic ions hydroxide (oh −) or oxide (o 2−) are classified. Alright, so remember, an ionic solid is an ionic compound. N2h4 c3h7nh2 co cuso4 this problem. Most ionic solids and molecular solids are insulators because of the negligible concentration of conduction. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure. Web this problem has been solved! In nature, the ordered arrangement of ionic solids gives. Web ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web ionic compounds usually form crystalline structures when solid. Web identify the ionic solid from the following options. Physical properties of ionic compounds. Rbi is called as rubidium iodide, which is made up of rubidium cation and. Which of the substances would form an ionic solid? Ionic solids are held together by the electrostatic attraction between the positive and negative ions. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions.
Web Ionic Compounds Are Solids That Typically Melt At High Temperatures And Boil At Even Higher Temperatures.
Web this problem has been solved! Rbi is called as rubidium iodide, which is made up of rubidium cation and. Web chemistry chemistry questions and answers which of the following forms an ionic solid? Sodium chloride is taken as a typical ionic compound.
Web Ionic Compounds Usually Form Crystalline Structures When Solid.
The ionic compound nacl forms when electrons from sodium atoms are transferred to chlorine atoms. Web ionic compounds include salts, oxides, hydroxides, sulphides, and the majority of inorganic compounds. Web precipitation reactions occur when cations and anions in aqueous solution combine to form an insoluble ionic solid. Web ionic compounds are compounds formed between a metal and nonmetal which have a crystalline lattice structure.
Physical Properties Of Ionic Compounds.
Which of the substances would form an ionic solid? Alright, so remember, an ionic solid is an ionic compound. Web complete ionic equations show dissolved ionic solids as separated ions. Ionic solids are made up of positive ion cation and negative ion anion.
Ionic Solids Are Held Together By The Electrostatic Attraction Between The Positive And Negative Ions.
In nature, the ordered arrangement of ionic solids gives. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. Most ionic solids and molecular solids are insulators because of the negligible concentration of conduction. You'll get a detailed solution from a subject matter expert that helps you learn core concepts.

.PNG)


.PNG)