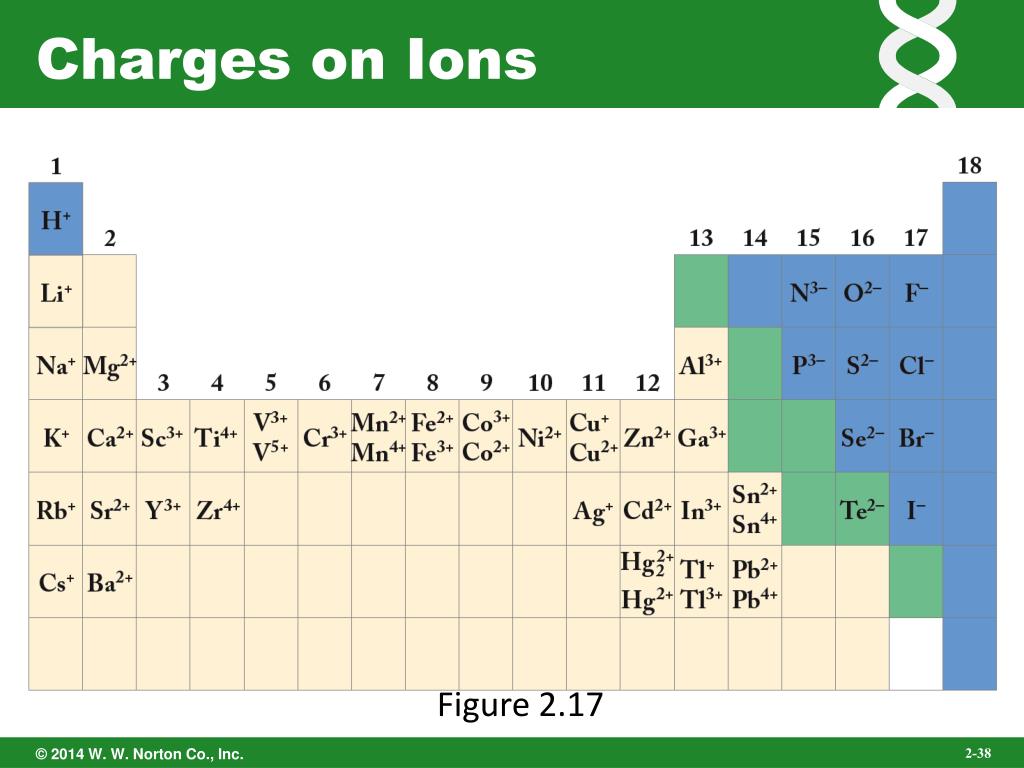
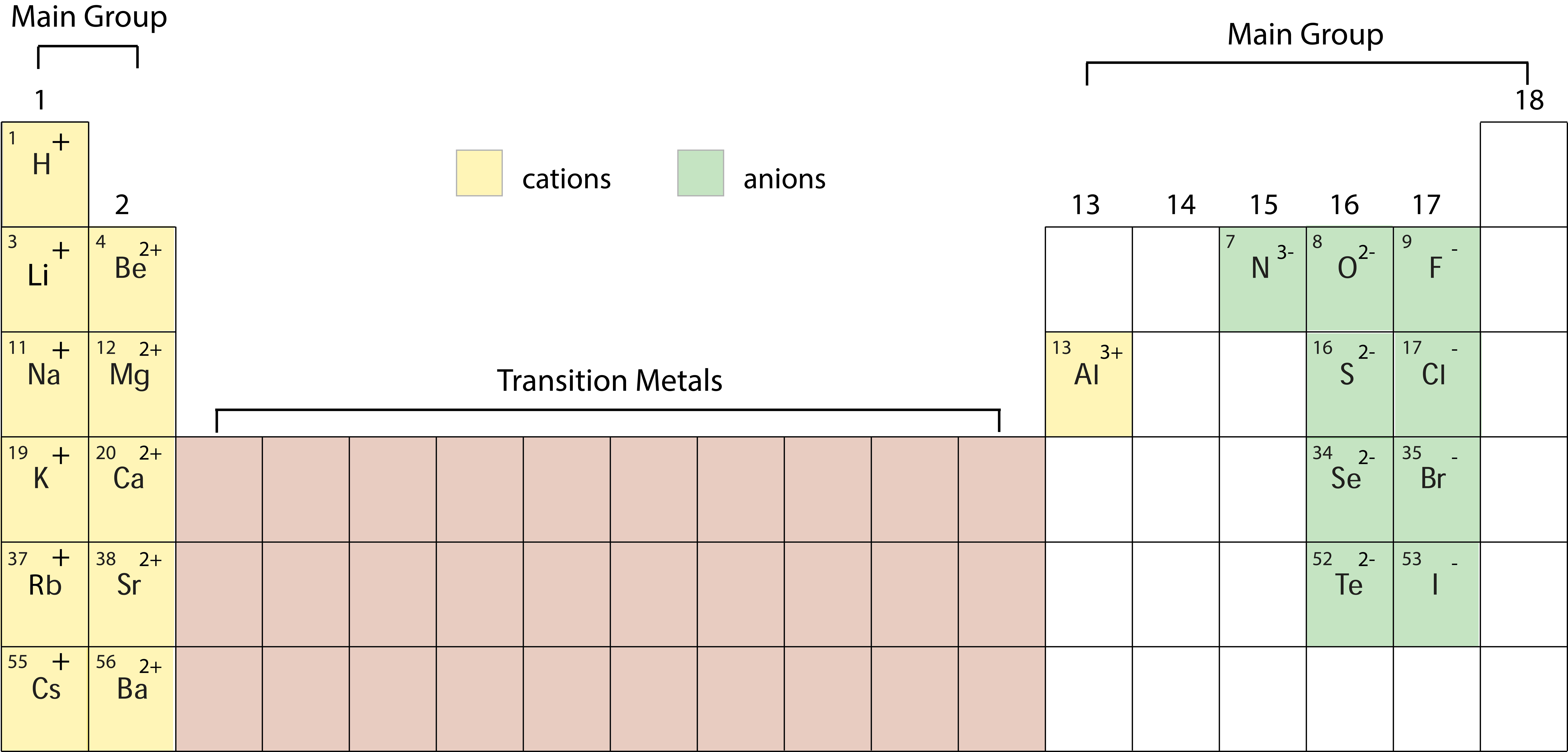
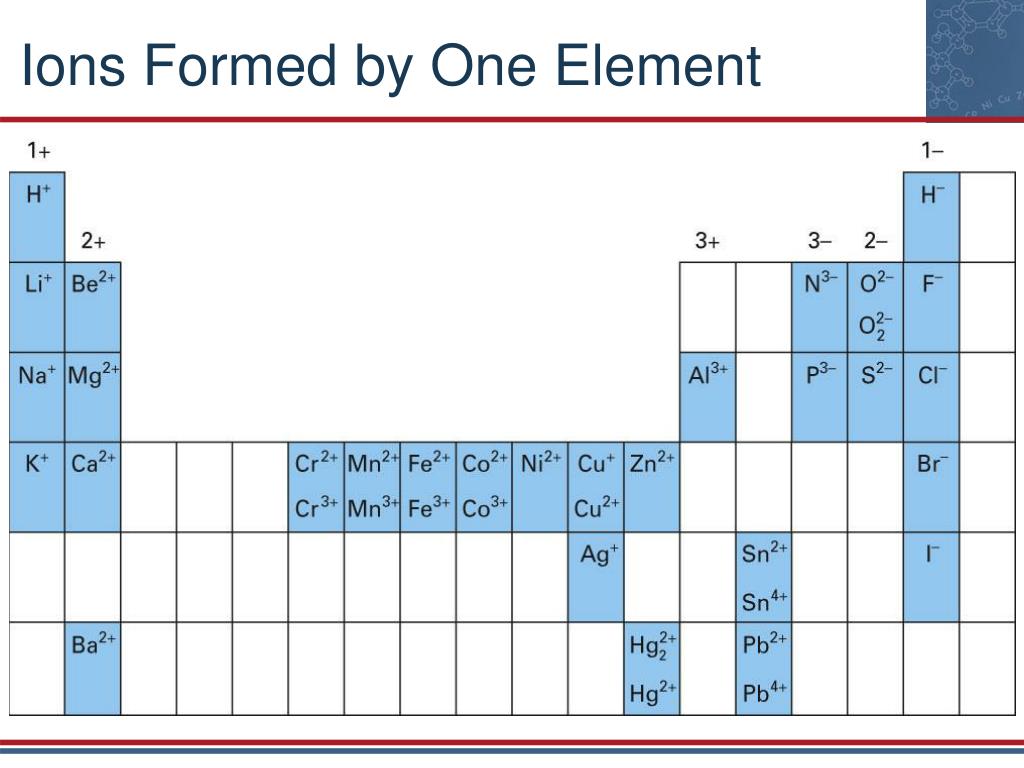
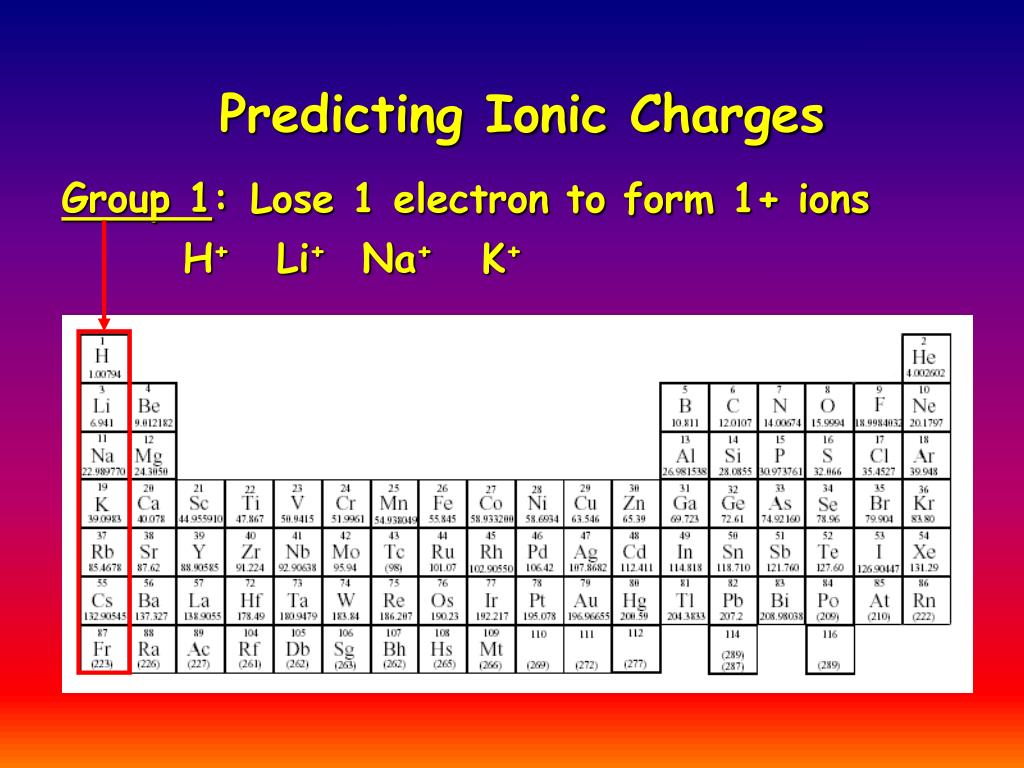
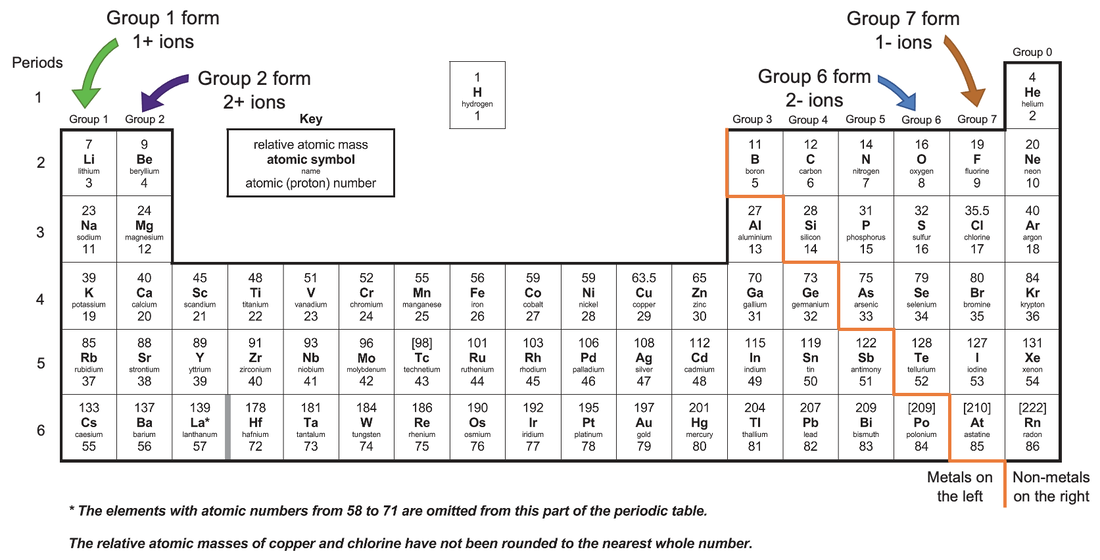
Which Group Tends To Form 1+ Ions - Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the. Halogens which group tends to form. Web which group tends to form 1+ ions? Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Alkaline earth metals which group tends to form 2+ ions? Web group 1, the alkali metals. Web neutral sodium atom on left has 11 protons and 11 electrons. Web which group tends to form 1+ ions? Group 2 elements form 2+ ions, and so on. Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when.
Chem Types of Chemical Equations Scientific Tutor
Moving from the far right to the left. Sodium ion on right has 11 protons and 10 electrons,. Group 2 elements form 2+ ions, and so on. Moving from the far right to the left on the periodic. Web which group forms 1 ions?
PPT Atoms, Ions, and Molecules Matter Starts Here PowerPoint
Web chemistry which group tends to form +1 ions ? So all alkali earth metals, group 2a, tend to form. The general outer electronic configuration of alkali metals are ns1,np0 if they. Moving from the far right to the left. Web which group tends to form 1+ ions?
Periodic Table Ions List Periodic Table Timeline
Web group 1 metals, the alkali metals, have the 1 valence electron and thus form m + ions when oxidized. Its because there's one valence electron in their outermost shell, they just lose that one. Group 2 elements form 2+ ions, and so on. Web which group forms 1 ions? Alkaline earth metals which group tends to form 2+ ions?
Periodic Table Which Groups Of Elements Tend To Form Positive Ions
Which group tends to form 1+ ions; Group 2 elements form 2+ ions, and so on. The 1st group (alkali metals) tends to form +1 ions. Web which group forms 1 ions? Web that is, group 1 elements form 1+ ions;
PreChemistry
Its because there's one valence electron in their outermost shell,. Which group tends to form +2 ions? Which group tends to form 2+ ions? Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Web chemistry which group tends to form +1 ions ?
PPT Chapter 6 Chemical Nomenclature PowerPoint Presentation, free
Web which group forms 1 ions? Its because there's one valence electron in their outermost shell, they just lose that one. Web group 1, the alkali metals. Web alkali metals are found in group 1 of the periodic table. Which group tends to form +1 ions?
Pin on Chem 105
Its because there's one valence electron in their outermost shell,. Alkaline earth metals which group tends to form 2+ ions? Web that is, group 1 elements form 1+ ions; Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when. Group 2 elements form 2+ ions, and so on.
PPT Atoms, Molecules, and Ions PowerPoint Presentation, free download
Web which group tends to form 1+ ions? Web that is, group 1 elements form 1+ ions; Web the alkali earth metals lose two electrons to resemble the next lowest noble gas; Web group 1, the alkali metals. The general outer electronic configuration of alkali metals are ns1,np0 if they.
C2 B) Ions from the Periodic Table AQA Combined Science Trilogy Elevise
Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the. Group 2 elements form 2+ ions, and so on. Chemistry middle school which group tends to form +1 ions ? Which group tends to form 2+ ions? The general outer electronic configuration of.
Ionic Compound Nomenclature Presentation Chemistry
Web which group tends to form 1+ ions? Web which group forms 1 ions? Web chemistry which group tends to form +1 ions ? Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Group 2 elements form 2+ ions, and so on.
Sodium ion on right has 11 protons and 10 electrons,. Which group tends to form +2 ions? Which group tends to form 1+ ions; Web which group tends to form 1+ ions? Alkaline earth metals which group tends to form 2+ ions? They will form ions with a 1+ charge. Web the alkali earth metals lose two electrons to resemble the next lowest noble gas; Web which group tends to form 1+ ions? Web the ions have the electronic structure of a noble gas (group 0 element), with a full outer shell for elements in groups 6 and 7, the. Web potassium, located directly beneath sodium in group 1, also forms +1 ions (k +) in its reactions, as do the remaining members of group 1: Group 2 elements form 2+ ions, and so on. Halogens which group tends to form. Web alkali metals are found in group 1 of the periodic table. Group 1, the alkali metals explanation: Group 1 metals, the alkali metals, have the 1 valence electron, and thus form m + ions when. Web that is, group 1 elements form 1+ ions; This is the group 1 elements. Chemistry middle school which group tends to form +1 ions ? Which group tends to form +1 ions? Group 2 elements form 2+ ions, and so on.
Halogens Which Group Tends To Form.
Web alkali metals are found in group 1 of the periodic table. They will form ions with a 1+ charge. Web which group forms 1 ions? Group 2 elements form 2+ ions, and so on.
The General Outer Electronic Configuration Of Alkali Metals Are Ns1,Np0 If They.
Web neutral sodium atom on left has 11 protons and 11 electrons. Web which group tends to form 1+ ions? Web the alkali earth metals lose two electrons to resemble the next lowest noble gas; The 1st group (alkali metals) tends to form +1 ions.
Web Group 1, The Alkali Metals.
Web which group tends to form 1+ ions? Moving from the far right to the left. Web that is, group 1 elements form 1+ ions; Moving from the far right to the left on the periodic.
Web Group 1 Metals, The Alkali Metals, Have The 1 Valence Electron And Thus Form M + Ions When Oxidized.
Group 2 elements form 2+ ions, and so on. Which group tends to form 2+ ions? The answer to this question is the alkali metals, which are located in group 1 of the. Which group tends to form +1 ions?





.PNG)