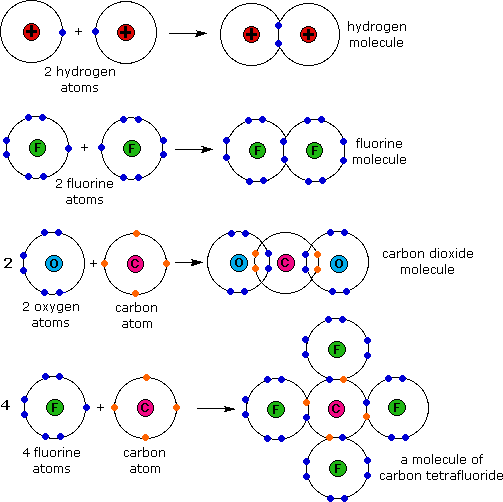
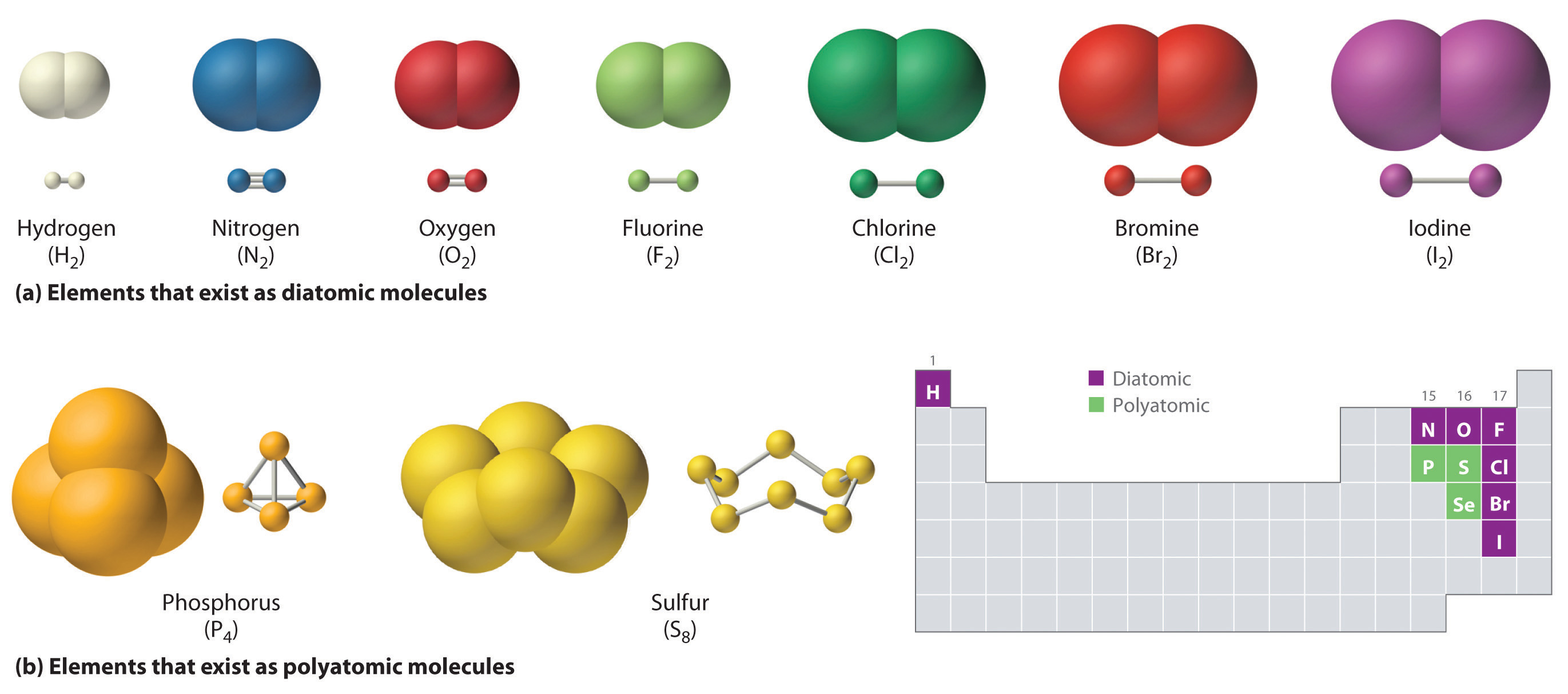
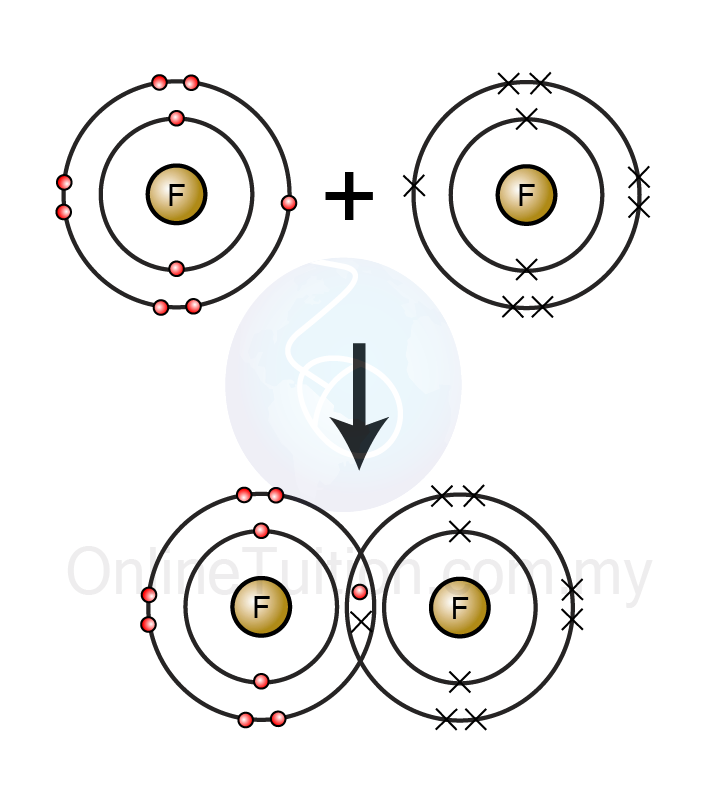
Which Element Will Most Likely Form Covalent Bonds With Fluorine - Web fluorine bonds with almost any element, both metals and nonmetals,. Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. Web one way to predict the type of bond that forms between two. Which pair of elements would most likely bond to form a covalently bonded compound? Web the flourine is a highly electronegative element, upon which different atoms will form bonds, such as:. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. This type of bonding occurs. Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Covalent bonding is the sharing of electrons between atoms.
5.2.1 Formation of Ion Revision.my
Web sodium and fluorine barium and chlorine phosphorus and oxygen magnesium and sulfur ; Choose the element that forms the covalent bond with the other element. Fluorine and the other halogens in. Web fluorine bonds with almost any element, both metals and nonmetals,. Web find an answer to your question an element that fluorine will form a covalent bond with.
PPT Covalent Bonds PowerPoint Presentation, free download ID6647183
Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Covalent bonding is the sharing of electrons between atoms. Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. This type of bonding occurs. Web find an.
Bonding Bonding and Chemical Interactions Training MCAT General
In this case, it is carbon that forms the covalent. Web the flourine is a highly electronegative element, upon which different atoms will form bonds, such as:. 1 covalent bonds in larger molecules how many covalent bonds are formed? Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web one, two, or.
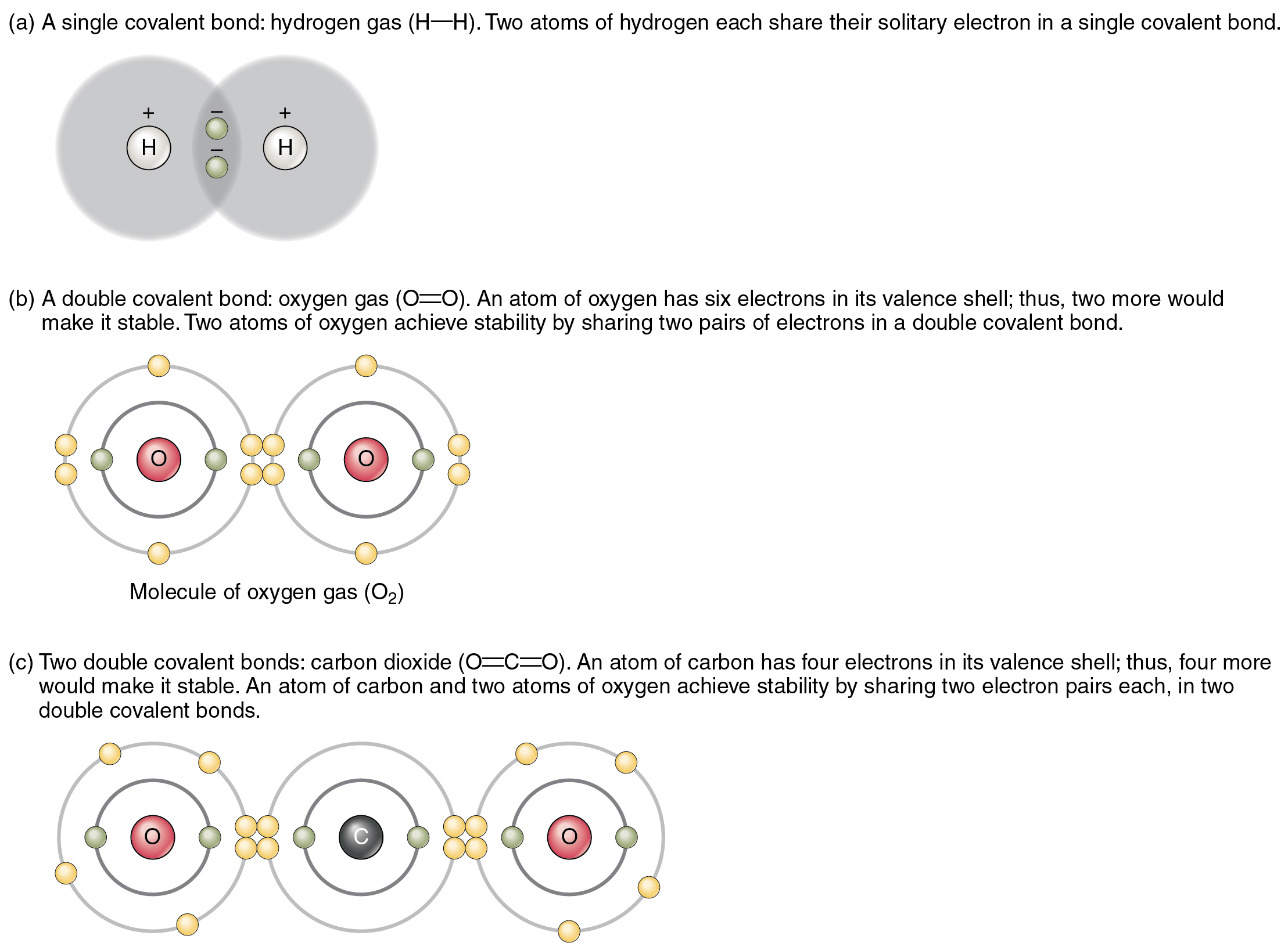
Covalent Bonding SPM Chemistry
Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. Web one way to predict the type of bond that forms between two. In this case, it is carbon that forms the covalent. Web nitrogen, oxygen, fluorine a chemical bond formed when two atoms share one pair of electrons.
Chemical Bonds · Anatomy and Physiology
Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web nitrogen, oxygen, fluorine a chemical bond formed when two atoms share one pair of electrons is a ________ bond; Choose the element that forms the covalent bond with the other element. Web the flourine is a highly electronegative element, upon which different.
Covalent Bonding (Biology) — Definition & Role Expii
Choose the element that forms the covalent bond with the other element. Web nitrogen, oxygen, fluorine a chemical bond formed when two atoms share one pair of electrons is a ________ bond; Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. Web fluorine bonds with almost any element,.
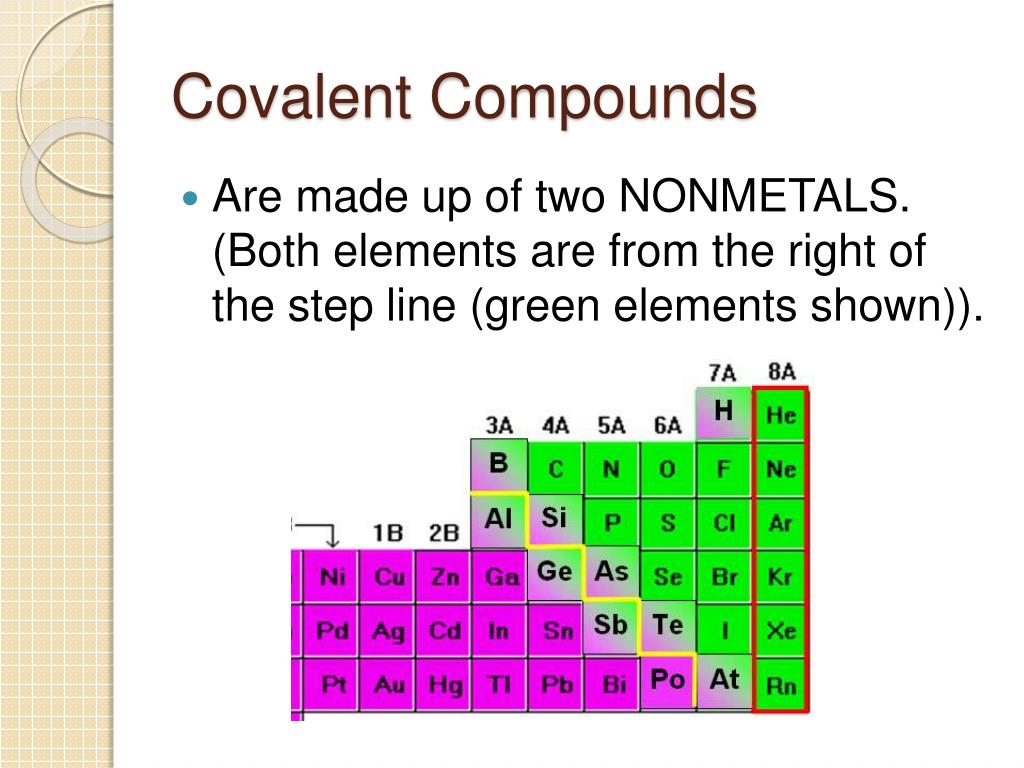
PPT Covalent Compounds PowerPoint Presentation, free download ID
Choose the element that forms the covalent bond with the other element. 1 covalent bonds in larger molecules how many covalent bonds are formed? In this case, it is carbon that forms the covalent. Web find an answer to your question an element that fluorine will form a covalent bond with. Fluorine and the other halogens in.
Ionic Bonding Presentation Chemistry
In this case, it is carbon that forms the covalent. 1 covalent bonds in larger molecules how many covalent bonds are formed? Web thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most. This type of bonding occurs. Web on the basis of their electron configurations, predict the formula of the.
EduMission May 2011
Web one way to predict the type of bond that forms between two. Which pair of elements would most likely bond to form a covalently bonded compound? Fluorine and the other halogens in. This type of bonding occurs. Choose the element that forms the covalent bond with the other element.
Molecules, Ions, and Chemical Formulas
Web the flourine is a highly electronegative element, upon which different atoms will form bonds, such as:. Which pair of elements would most likely bond to form a covalently bonded compound? Covalent bonding is the sharing of electrons between atoms. Web on the basis of their electron configurations, predict the formula of the simple binary ionic compounds likely to form.
Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. Web thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most. Web nitrogen, oxygen, fluorine a chemical bond formed when two atoms share one pair of electrons is a ________ bond; Web fluorine will form covalent and ionic bonds. Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table. Web one way to predict the type of bond that forms between two. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. In this case, it is carbon that forms the covalent. Web on the basis of their electron configurations, predict the formula of the simple binary ionic compounds likely to form when the. This type of bonding occurs. Choose the element that forms the covalent bond with the other element. Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. 1 covalent bonds in larger molecules how many covalent bonds are formed? Web the flourine is a highly electronegative element, upon which different atoms will form bonds, such as:. Fluorine and the other halogens in. Covalent bonding is the sharing of electrons between atoms. Which pair of elements would most likely bond to form a covalently bonded compound? Web sodium and fluorine barium and chlorine phosphorus and oxygen magnesium and sulfur ; Web fluorine bonds with almost any element, both metals and nonmetals,. Web find an answer to your question an element that fluorine will form a covalent bond with.
In This Case, It Is Carbon That Forms The Covalent.
Web thus, the nonmetals, which lie in the upper right, tend to have the highest electronegativities, with fluorine the most. This type of bonding occurs. Web the high electronegativity of fluorine means that it forms a single electron pair bond polar bond with a high ionic character. Web one, two, or three pairs of electrons may be shared between atoms, resulting in single, double, or triple bonds, respectively.
Web Nitrogen, Oxygen, Fluorine A Chemical Bond Formed When Two Atoms Share One Pair Of Electrons Is A ________ Bond;
Web sodium and fluorine barium and chlorine phosphorus and oxygen magnesium and sulfur ; Web oxygen and other atoms in group 6a (16) obtain an octet by forming two covalent bonds. Web predict the number of covalent bonds formed based on the elements involved and their position on the periodic table. Covalent bonding is the sharing of electrons between atoms.
Web One Way To Predict The Type Of Bond That Forms Between Two.
Web on the basis of their electron configurations, predict the formula of the simple binary ionic compounds likely to form when the. 1 covalent bonds in larger molecules how many covalent bonds are formed? Fluorine and the other halogens in. Which pair of elements would most likely bond to form a covalently bonded compound?
Web Fluorine Bonds With Almost Any Element, Both Metals And Nonmetals,.
Web the flourine is a highly electronegative element, upon which different atoms will form bonds, such as:. Choose the element that forms the covalent bond with the other element. Web of course, fluoride ions, f −, are very common in chemistry, and here the fluorine nucleus has achieved an 8. Web find an answer to your question an element that fluorine will form a covalent bond with.

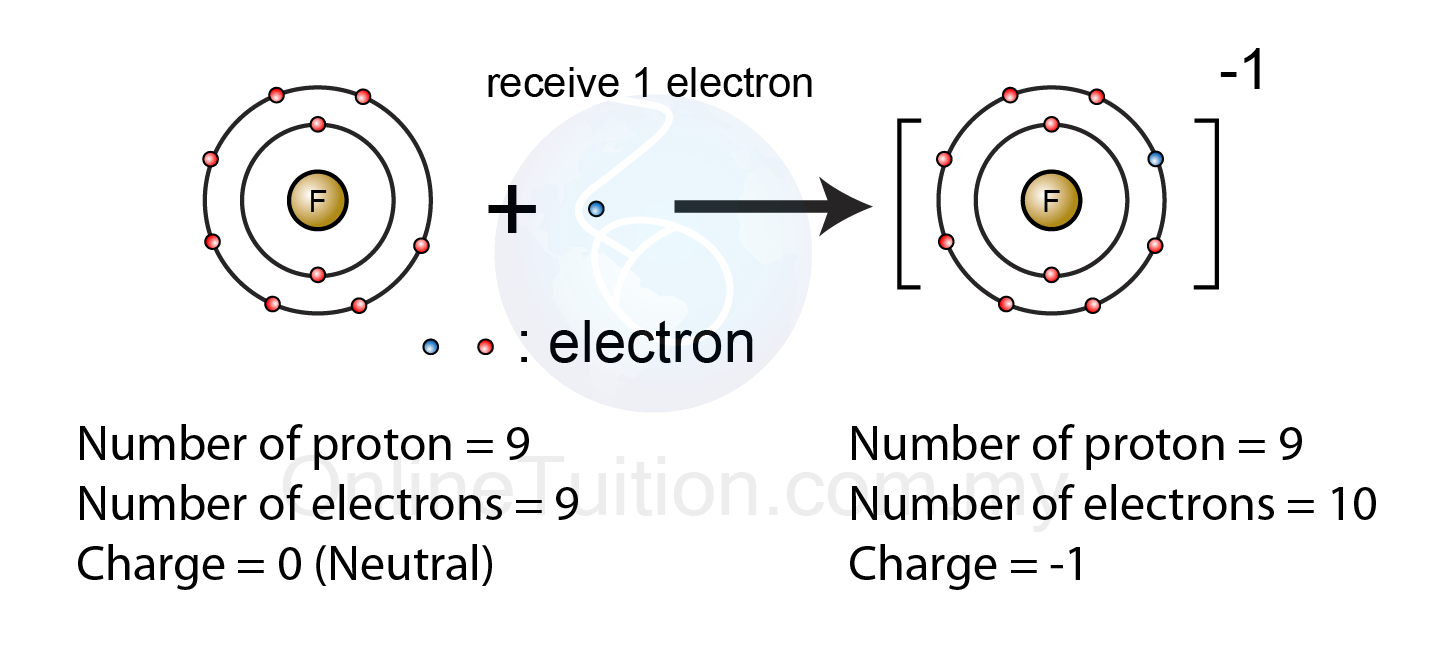

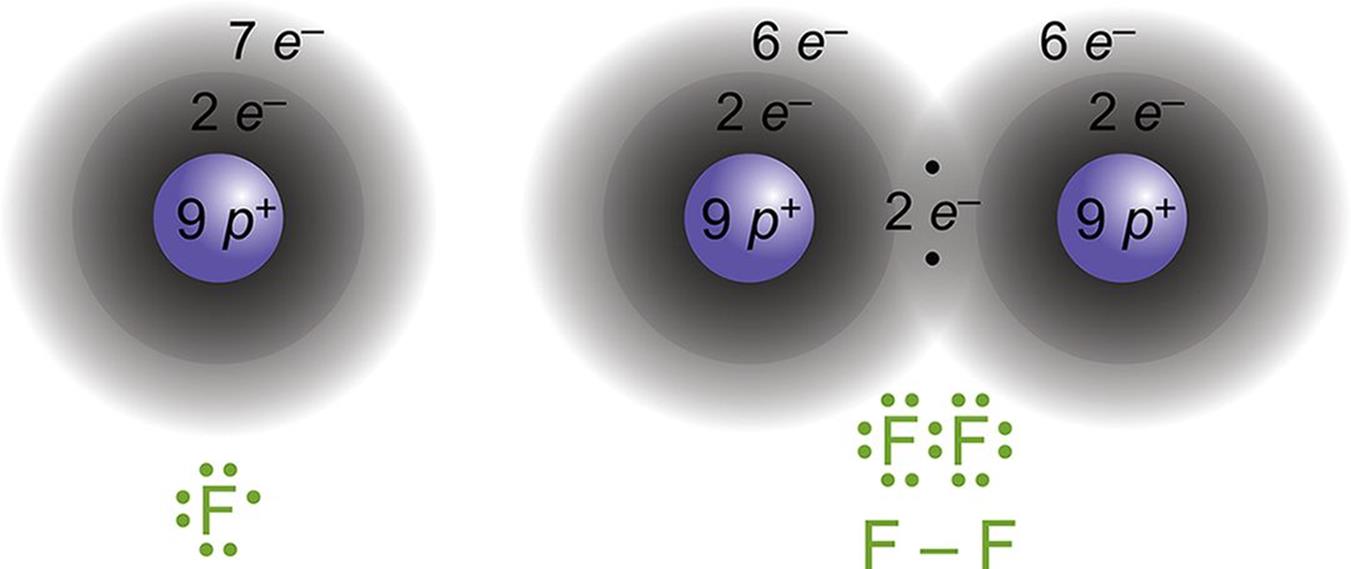

.PNG)