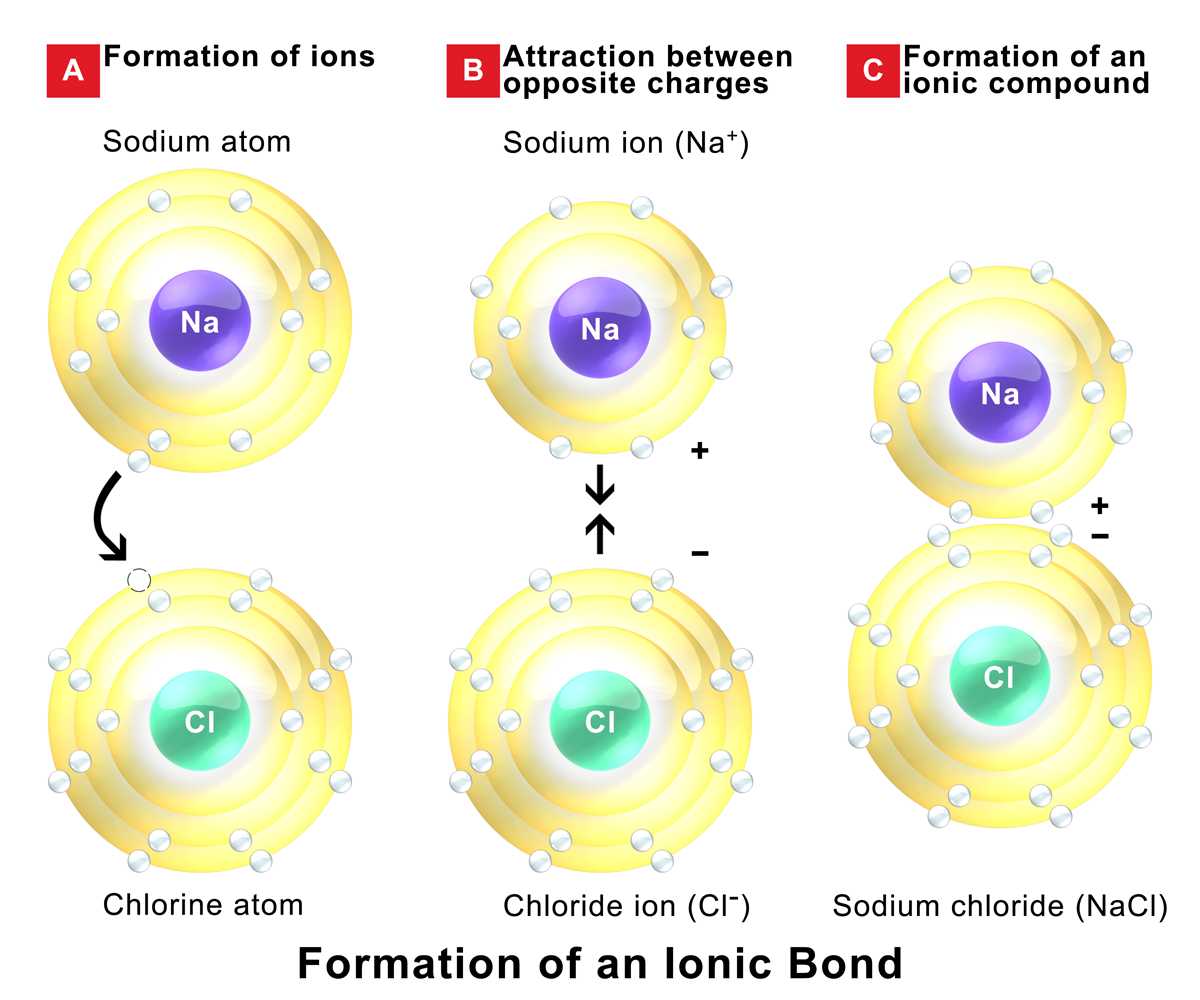
Which Best Describes How An Ionic Bond Forms - 3/7/2022 wiki user ∙ 7y ago study now see answer (1). Web what best describes how an ionic bond forms? The transfer of electrons forms strong bonds between ions. Web which statement best describes how an ionic bond forms? A bond’s strength describes how strongly each. Web an ionic bond, also called an electrovalent bond, is a chemical bond formed by electrostatic interaction between two. Web which statement best describes how an ionic bond forms? They are formed as a result of the electrostatic attraction. Web ionic bond strength and lattice energy. Web examples of ionic bonds.
Ionic Bond Definition, Types, Properties & Examples
An ionic compound is stable because of the electrostatic attraction between its. Web in our description of ionic bonding, we will explore the questions of what determines the bond length and bond strength of an ionic. Web what best describes how an ionic bond forms? Web what best describes how an ionic bond forms? The transfer of electrons forms strong.
What Best Describes How an Ionic Bond Forms
An ionic compound is stable because of the electrostatic attraction between its. The transfer of electrons forms strong bonds between ions. Web which statement best describes how an ionic bond forms? Web the best description of how an ionic bond forms is one atom pulls an electron from another atom. They form as a result of electrostatic attraction between.
Ionic Bonding in a Solid Sodium Chloride Crystal Stock Vector
The transfer of electrons forms strong bonds between ions. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent. They form as a result of electrostatic attraction between. Web elements an element is a pure chemical substance that cannot be broken down into another chemical substance. A metal and nonmetal react.
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare
A bond’s strength describes how strongly each. Web elements an element is a pure chemical substance that cannot be broken down into another chemical substance. In nacl, the ionic bond is formed between the metal ion, na+, and. Ionic bonding refers to the complete. Web in our description of ionic bonding, we will explore the questions of what determines the.
ionic bond Definition, Properties, Examples, & Facts Britannica
Web ionic bonds are one of the two main types of chemical bonds. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Ionic bonding refers to the complete. Web ionic bonds are interatomic or intramolecular bonds. Web ionic bonds typically form when the difference in the electronegativities of the two atoms.
PreChemistry
Web which statement best describes how an ionic bond forms? An ionic compound is stable because of the electrostatic attraction between its. Web ionic bonds are interatomic or intramolecular bonds. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. They form as a result of electrostatic attraction between.
Chemical Bonds
Web ionic bond strength and lattice energy. Web ionic bonds are interatomic or intramolecular bonds. In nacl, the ionic bond is formed between the metal ion, na+, and. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent. 3/7/2022 wiki user ∙ 7y ago study now see answer (1).
Ionic Compounds Ionic bonds, Properties, Formation, Examples, Videos
Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent. Web the best description of how an ionic bond forms is one atom pulls an electron from another atom. They are formed as a result of the electrostatic attraction. Ionic bonds form between two or more atoms by the transfer of.
Ionic Bond Definition, Types, Properties & Examples
Web which statement best describes how an ionic bond forms? Web the best description of how an ionic bond forms is one atom pulls an electron from another atom. They form as a result of electrostatic attraction between. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web which statement best.
Ionic Bond Definition, Types, Properties & Examples
Web ionic bond strength and lattice energy. Web in our description of ionic bonding, we will explore the questions of what determines the bond length and bond strength of an ionic. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. Web what best describes how an ionic bond forms? The transfer.
Web the best description of how an ionic bond forms is one atom pulls an electron from another atom. Web ionic bonds are one of the two main types of chemical bonds. Web elements an element is a pure chemical substance that cannot be broken down into another chemical substance. Web what best describes how an ionic bond forms? Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. Web what best describes how an ionic bond forms? Web examples of ionic bonds. Web which statement best describes how an ionic bond forms? The transfer of electrons forms strong bonds between ions. 3/7/2022 wiki user ∙ 7y ago study now see answer (1). Web ionic bond refers to a type of chemical bond which generates two oppositely charged ions. Web ionic bonds are interatomic or intramolecular bonds. Ionic bonds form between two or more atoms by the transfer of one or more electrons between atoms. An ionic compound is stable because of the electrostatic attraction between its. The transfer of electrons forms strong bonds between ions. In nacl, the ionic bond is formed between the metal ion, na+, and. Web an ionic bond, also called an electrovalent bond, is a chemical bond formed by electrostatic interaction between two. Web there are many types of chemical bonds and forces that bind molecules together. Ionic bonding refers to the complete. Web ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent.
They Form As A Result Of Electrostatic Attraction Between.
Ionic bonding refers to the complete. Web ionic bond strength and lattice energy. Web in our description of ionic bonding, we will explore the questions of what determines the bond length and bond strength of an ionic. Web which statement best describes how an ionic bond forms?
Ionic Bonds Form Between Two Or More Atoms By The Transfer Of One Or More Electrons Between Atoms.
Web examples of ionic bonds. Web elements an element is a pure chemical substance that cannot be broken down into another chemical substance. Web ionic bonds are one of the two main types of chemical bonds. Web what best describes how an ionic bond forms?
Web An Ionic Bond, Also Called An Electrovalent Bond, Is A Chemical Bond Formed By Electrostatic Interaction Between Two.
The transfer of electrons forms strong bonds between ions. Web ionic bonding is the complete transfer of valence electron (s) between atoms and is a type of chemical bond that. Web ionic bonds are interatomic or intramolecular bonds. Web which statement best describes how an ionic bond forms?
A Bond’s Strength Describes How Strongly Each.
The transfer of electrons forms strong bonds between ions. Web the best description of how an ionic bond forms is one atom pulls an electron from another atom. An ionic compound is stable because of the electrostatic attraction between its. Web what best describes how an ionic bond forms?



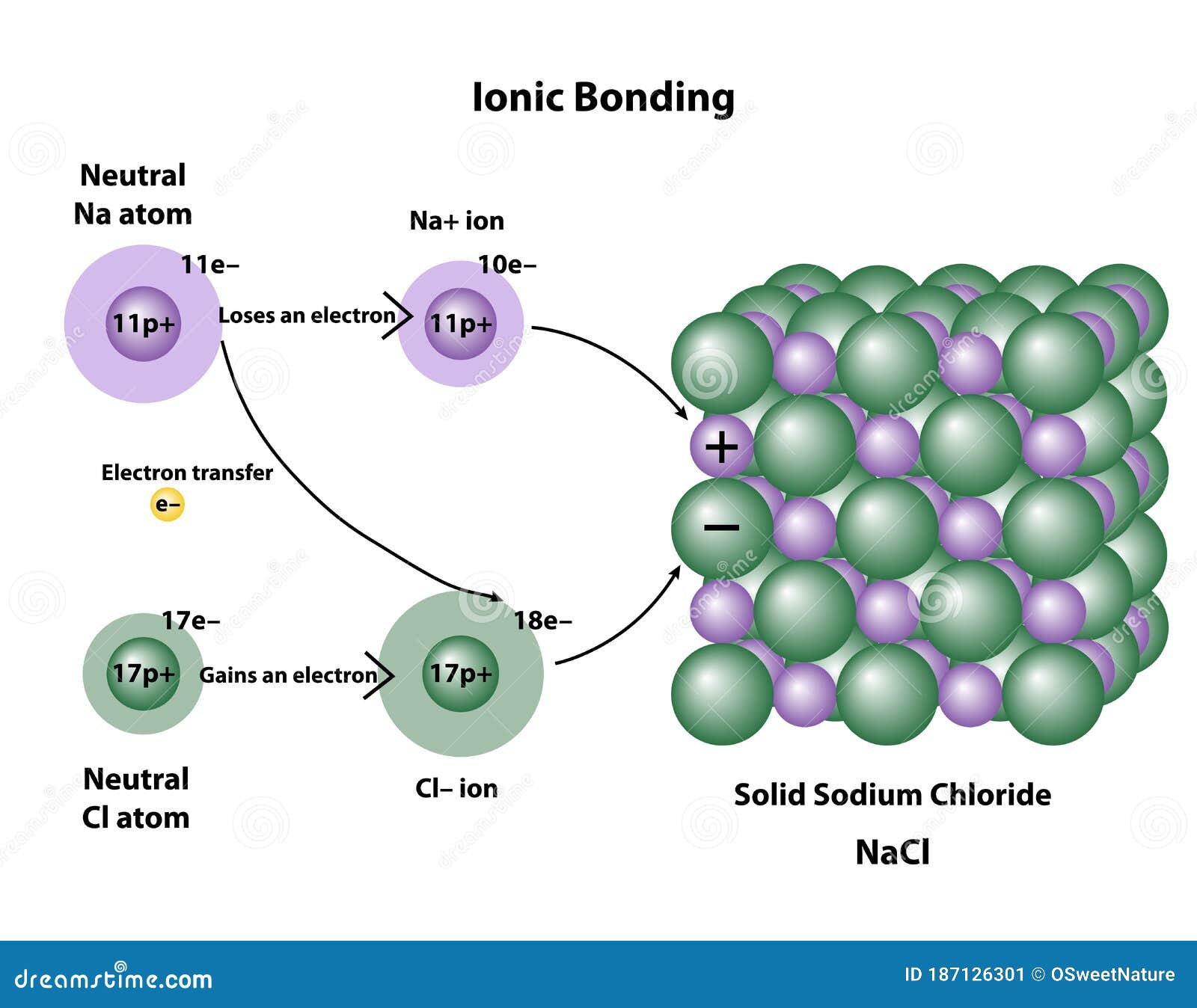
.PNG)

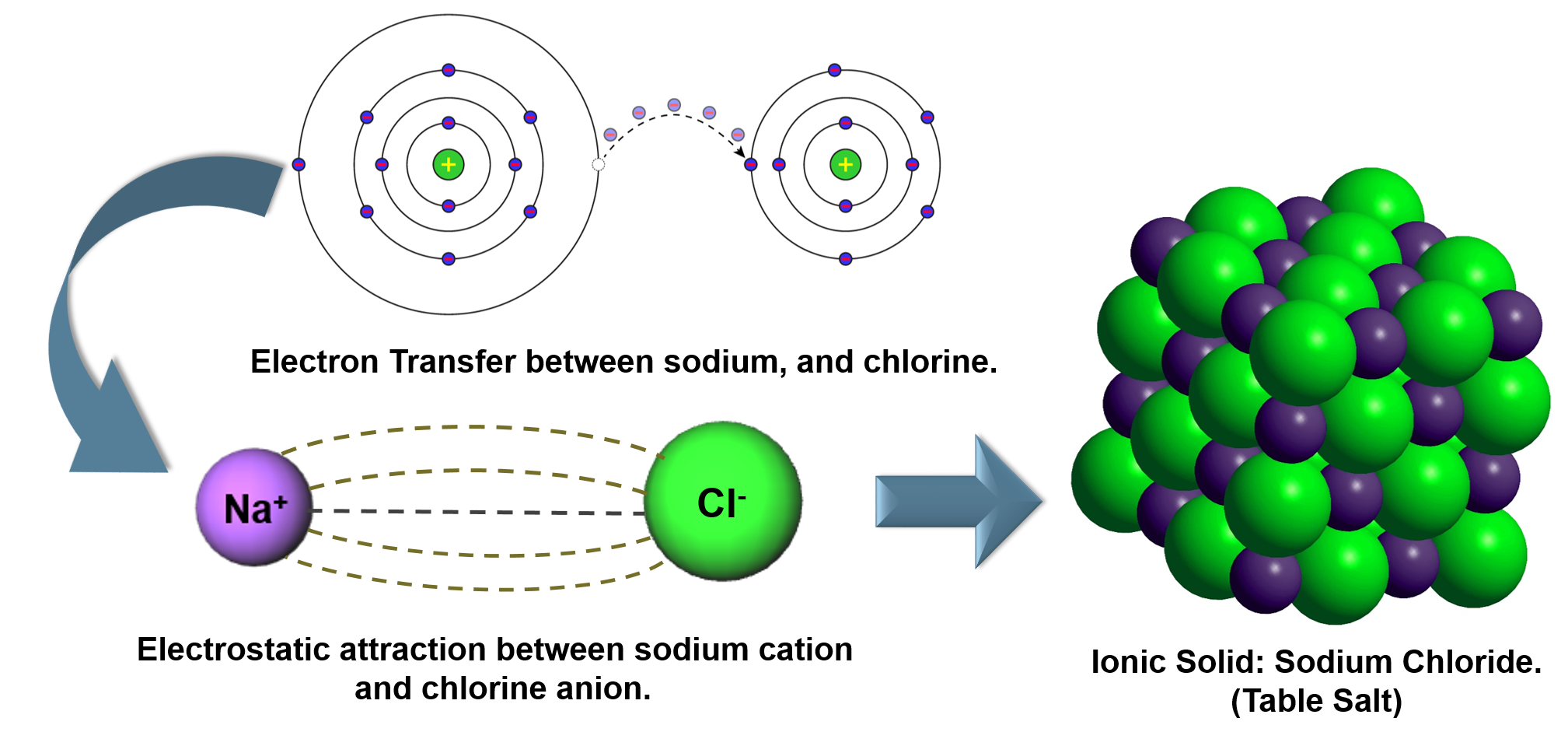
.PNG)