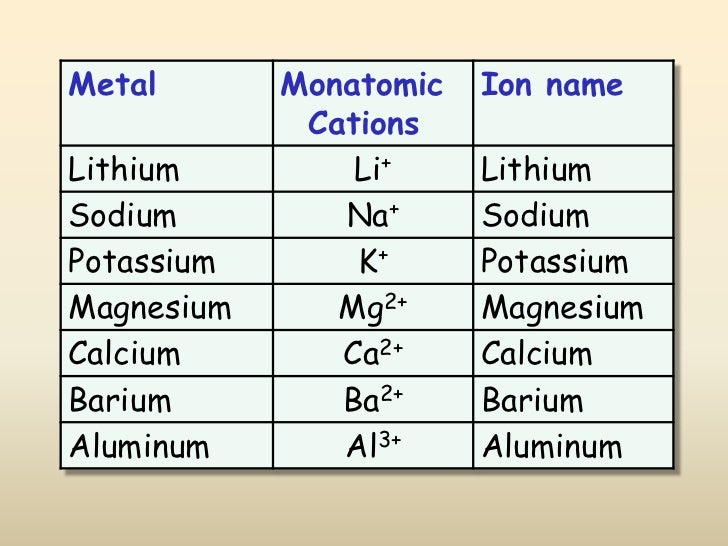
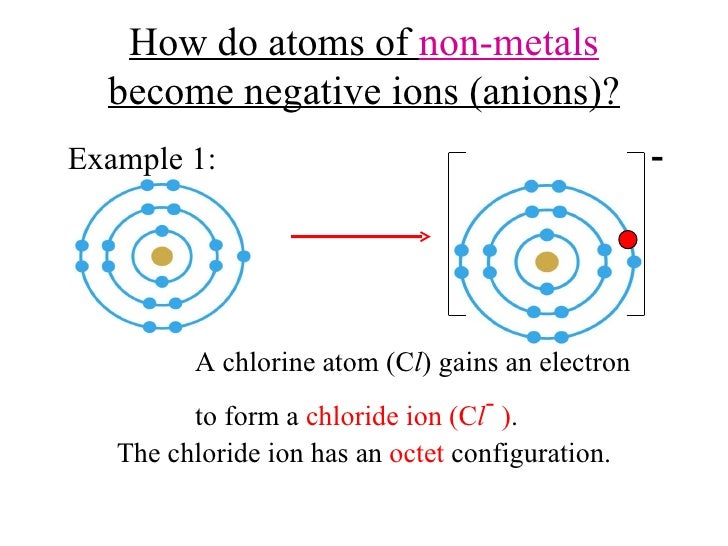
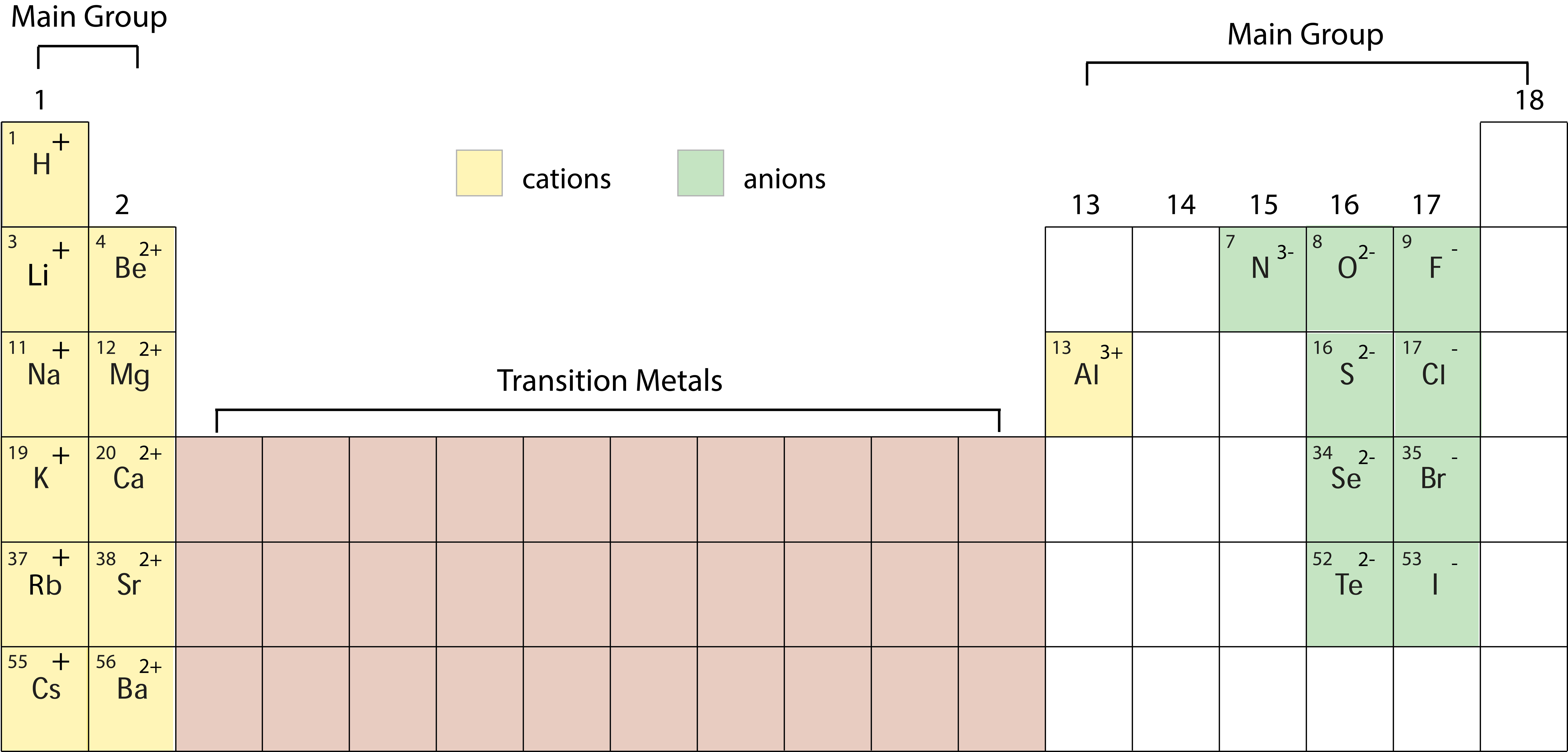
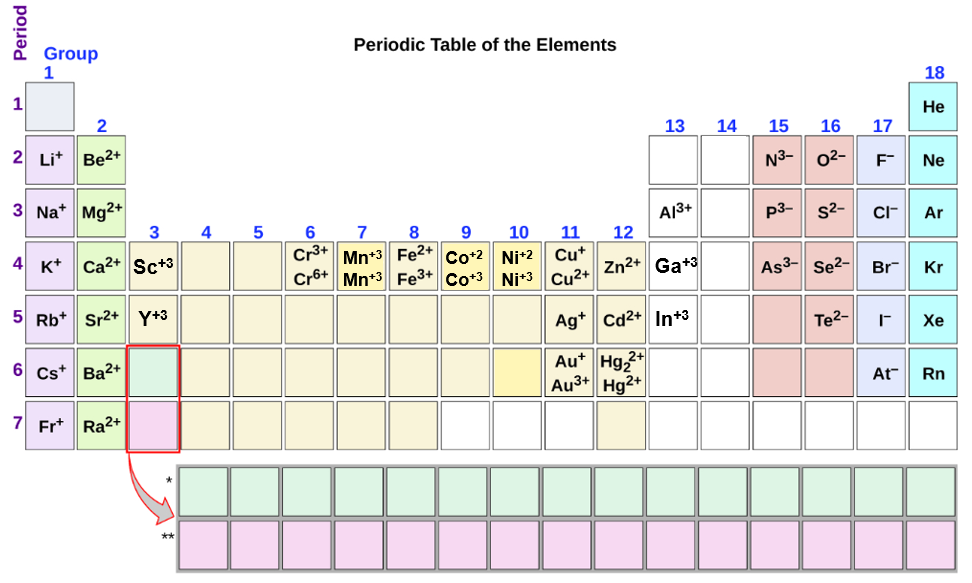
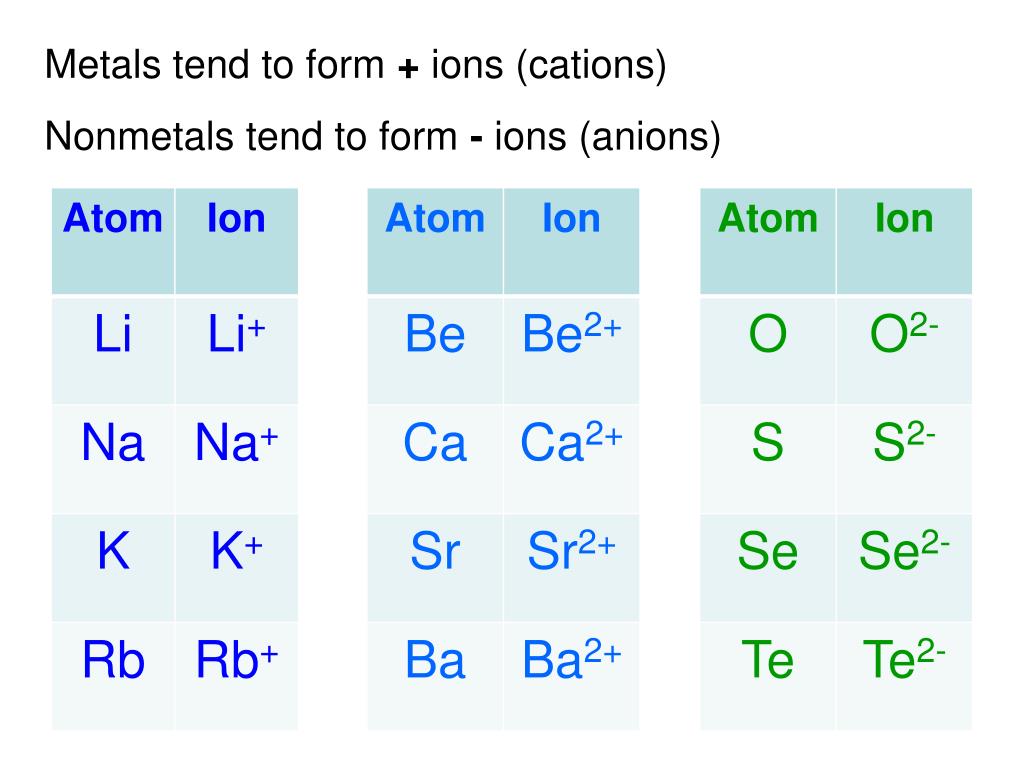
When Metals Form Ions They Tend To Do So By - Web in the chemistry of the transition elements, the 4s orbital behaves as the outermost, highest energy orbital. Web answer (1 of 4): Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic. When a group iia atom forms an ion, what charge does it have?. At an atomic level, the valence. Web metal atoms lose electrons from their outer shell when they form ions: They do it because they. Metals tend to form cations,. This is actually one of the chemical properties of metals and nonmetals:
Chem matters ch6_ionic_bond
Web metals form ions by losing electrons. Therefore, they tend to give away atoms and become positively charged. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. Web metals have tendency to loose electron and forms cations as. Web when metals form ions, they tend to.
Ions
Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web metals form ions by losing electrons. And thus metals tend to form positive ions. They do it because they. Web this is actually one of the chemical properties of metals and nonmetals:
Ionic bonding
Web metal atoms lose electrons from their outer shell when they form ions: This is actually one of the chemical properties of metals and nonmetals: Metals tend to form cations,. Web metals have tendency to loose electron and forms cations as. They do it because they.
Chem matters ch6_ionic_bond
And thus metals tend to form positive ions. The ions are positive, because they have more protons. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web answer (1 of 4):
Chem matters ch6_ionic_bond
At an atomic level, the valence. The ions are positive, because they have more protons. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Therefore, they tend to give away atoms and become positively charged. M + δ → m 2+ + 2e−.
PreChemistry
They do it because they have few electrons in their outermost shells. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. We know that nonmetals tend to form negative ions. Losing electrons and forming positive ionsexplanation:metals form ions by losing electrons. Web this is actually one.
2.6 Ionic Compounds and Formulas Chemistry LibreTexts
Web compounds that contain ions are called ionic compounds. Therefore, they tend to give away atoms and become positively charged. The reason nonmetals tend to form negative. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. This is actually one of the chemical properties of metals.
Chem matters ch6_ionic_bond
When a group iia atom forms an ion, what charge does it have?. We know that nonmetals tend to form negative ions. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. And thus metals tend to form positive ions. At an atomic level, the valence.
PPT THE NATURE OF MATERIALS PowerPoint Presentation, free download
When a group iia atom forms an ion, what charge does it have?. At an atomic level, the valence. Web metal atoms lose electrons from their outer shell when they form ions: Web this is actually one of the chemical properties of metals and nonmetals: Web in the chemistry of the transition elements, the 4s orbital behaves as the outermost,.
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free
Therefore, they tend to give away atoms and become positively charged. Web this is actually one of the chemical properties of metals and nonmetals: Metals tend to form cations,. The reason nonmetals tend to form negative. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose.
They do it because they have few electrons in their outermost shells. Metals tend to form cations,. Web metals have tendency to loose electron and forms cations as. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Web metals form ions by losing electrons. Web answer (1 of 4): Metals tend to form cations, while nonmetals tend to form anions. They do it because they. Web with the exception of hydrogen, all elements that form positive ions by losing electrons during chemical reactions are. Ionic compounds generally form from metals. Web this is actually one of the chemical properties of metals and nonmetals: Web when metals form ions, they tend to do so by losing their electrons to gain a positive charge. M + δ → m 2+ + 2e−. When a group iia atom forms an ion, what charge does it have?. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. Web compounds that contain ions are called ionic compounds. Web metals need to give away atoms. Therefore, they tend to give away atoms and become positively charged. Web in general, when metals form an ion, it is easier for them to lose electrons to attain a stable noble gas electronic. This is actually one of the chemical properties of metals and nonmetals:
Web When Metals Form Ions, They Tend To Do So By Losing Their Electrons To Gain A Positive Charge.
Metals tend to form cations,. They do it because they have few electrons in their outermost shells. Metals tend to form cations, while nonmetals tend to form anions. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose.
When A Group Iia Atom Forms An Ion, What Charge Does It Have?.
They do it because they. This is actually one of the chemical properties of metals and nonmetals: Web metals have tendency to loose electron and forms cations as. Web metal atoms lose electrons from their outer shell when they form ions:
Web With The Exception Of Hydrogen, All Elements That Form Positive Ions By Losing Electrons During Chemical Reactions Are.
Web metals form ions by losing electrons. The reason nonmetals tend to form negative. We know that nonmetals tend to form negative ions. Web answer (1 of 4):
Web Metals Need To Give Away Atoms.
Web compounds that contain ions are called ionic compounds. Web so this question we are talking about why medals like to form cat ions and non models like the form and ions. Web this is actually one of the chemical properties of metals and nonmetals: Ionic compounds generally form from metals.