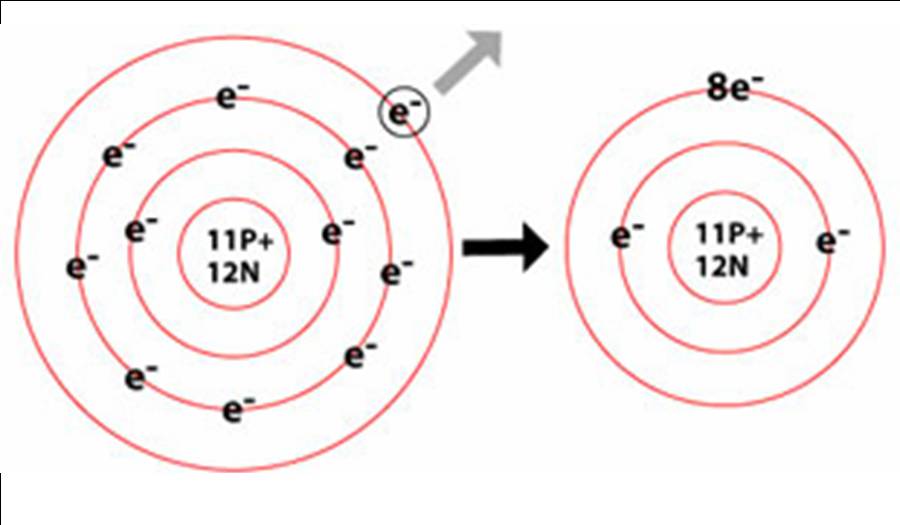
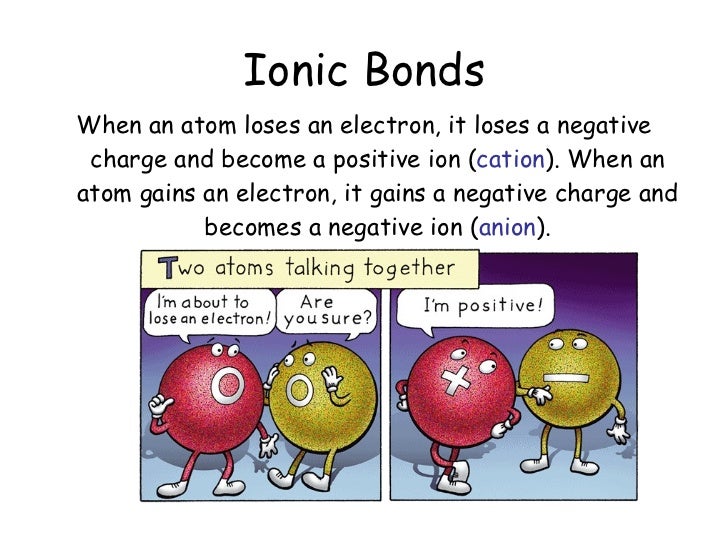
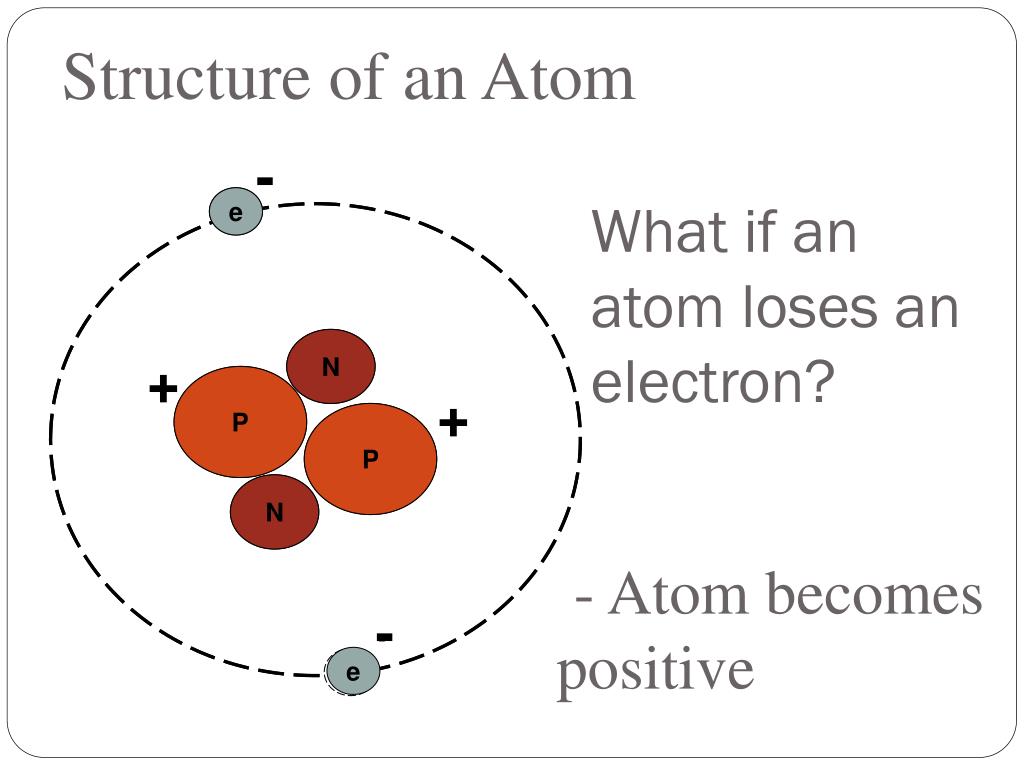
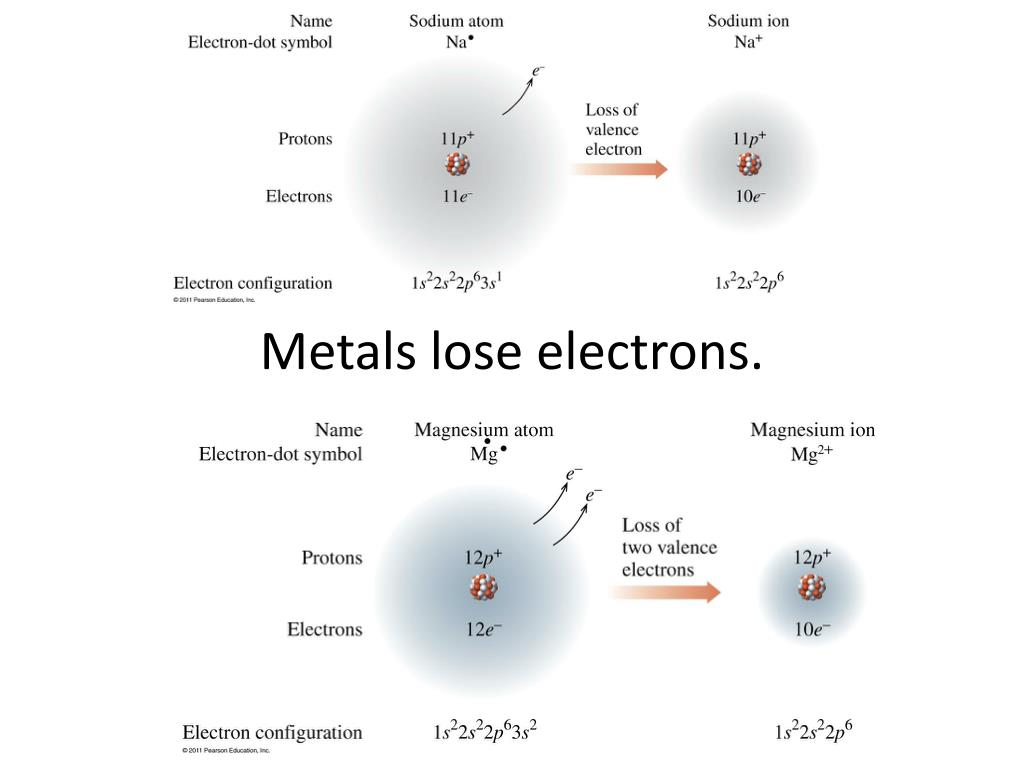
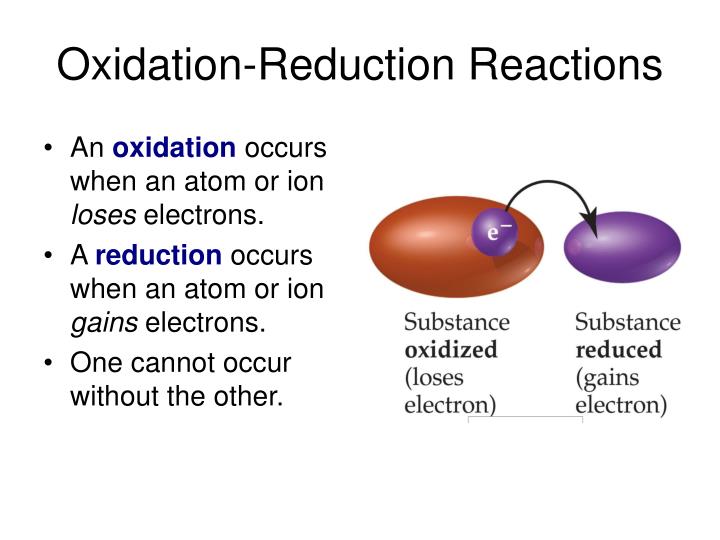
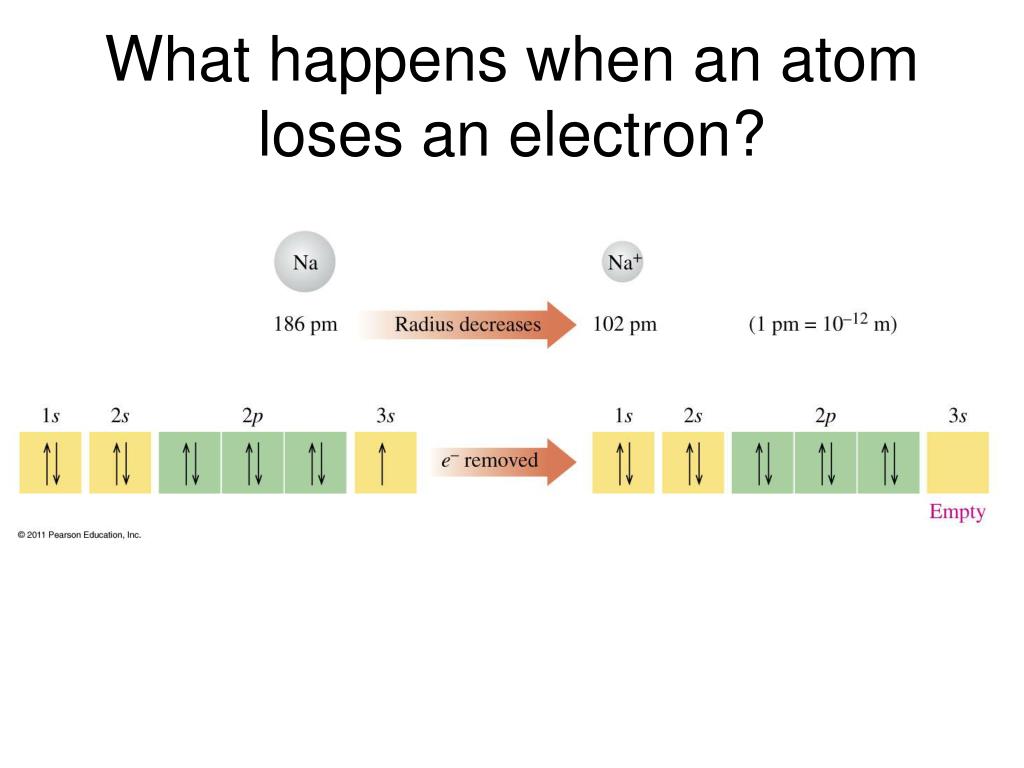
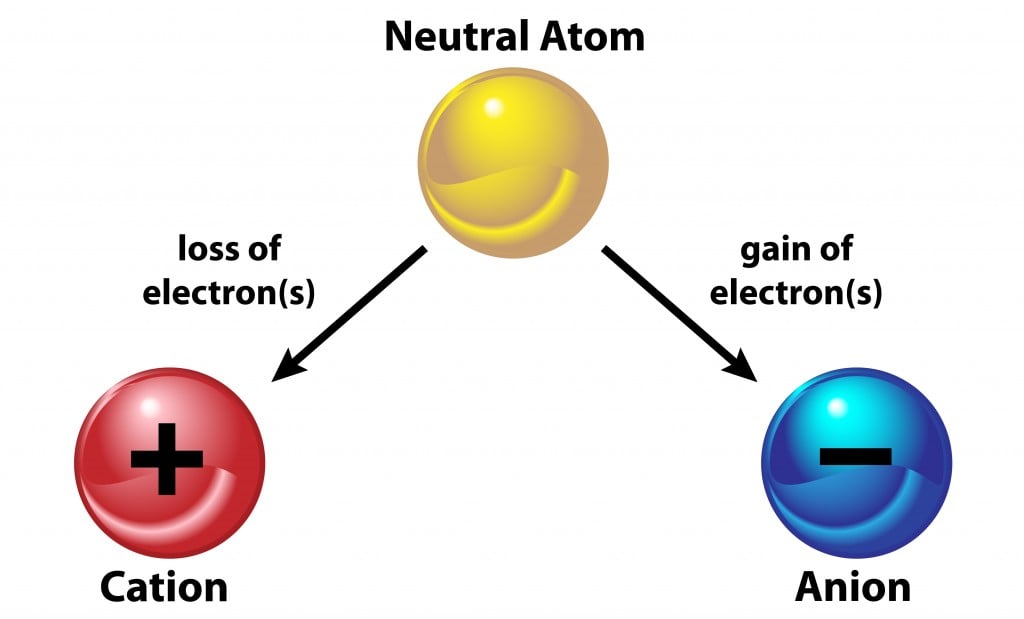
When An Atom Loses An Electron It Forms A - Web when forming a cation, an atom of a main group element tends to lose all of its valence electrons, thus assuming the electronic. When an atom gains an electron, it. Ions form when an atom either gains or loses electrons. Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. Metal atoms lose electrons to form positively charged ions. Web in an atom, the potential energy from moving the electron in the coulombic potential (the attraction of an. Web (a) cation when an atom loses electrons, it becomes a positively charged ion and it is called a cation. If it loses electrons, it becomes a positive ion,. The cation of sodium(na +). To obtain a full outer shell:
Hl Life Science Final Exam ProProfs Quiz
Web in an atom, the potential energy from moving the electron in the coulombic potential (the attraction of an. Web thus, it is concluded that when an atom with no charge loses two electrons, it becomes a positive ion. Web when an atom loses an electron it gains a positive charge and is called a cation. Web solution verified by.
Bonding
Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. When an atom gains an electron, it. Web the marcus model forms the foundation for all modern discussion of electron transfer (et). Web study with quizlet and memorize flashcards containing terms like when.
PPT Chemistry Midterm Review PowerPoint Presentation ID1988469
Web when forming a cation, an atom of a main group element tends to lose all of its valence electrons, thus assuming the electronic. The correct option is b gains or loses electrons. Web if an atom loses or gains electrons, it will become a positively or negatively charged particle, called an ion. If the atom gains one or more.
PPT UNIT 2 Basic Chemistry PowerPoint Presentation, free download
Metal atoms lose electrons to form positively charged ions. Loss of electrons from atom results in the formation of an ion known as a cation. Web ions form when atoms lose or gain electrons. The correct option is b gains or loses electrons. Web atoms that lose electrons acquire a positive charge as a result because they are left with.
PPT Chemistry 120 PowerPoint Presentation, free download ID6788469
When an atom gains an electron, it. Web if an atom loses or gains electrons, it will become a positively or negatively charged particle, called an ion. When an atom loses or gains an electron, ions are formed. Web when an atom loses an electron it gains a positive charge and is called a cation. An atom loses electrons to.
PPT Recap PowerPoint Presentation ID4504343
Web solution verified by toppr correct option is a) when an atom loses an e − nuclear charge increases due to the greater number of. When an atom gains an electron it gains a. When an atom with less than 4. When an atom loses an electron, it becomes a cation (positive ion). Web ions form when atoms lose or.
PPT Chapter 2 PowerPoint Presentation, free download ID9313297
Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. When an atom loses or gains an electron, ions are formed. Web the marcus model forms the foundation for all modern discussion of electron transfer (et). To obtain a full outer shell: When.
Formation of Ion SPM Chemistry
Web study with quizlet and memorize flashcards containing terms like when an atom loses an electron, it forms a(n), the charge on a. An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted. When an atom gains an electron, it. If it loses electrons, it becomes a positive ion,. Web when.
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint
When an atom gains an electron, it. Web study with quizlet and memorize flashcards containing terms like when an atom loses an electron, it forms a(n), the charge on a. Loss of electrons from atom results in the formation of an ion known as a cation. When an atom with less than 4. If it loses electrons, it becomes a.
Octet Rule Definition, Explanation, Exceptions and Examples
Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. When an atom gains an electron it gains a. Web if an atom.
Loss of electrons from atom results in the formation of an ion known as a cation. Web when an atom loses an electron it gains a positive charge and is called a cation. Web solution the correct option is a cation, anion cation is the name given to the positive ion of an element when it loses electrons. If it loses electrons, it becomes a positive ion,. When an atom gains an electron it gains a. The cation of sodium(na +). Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. Metal atoms lose electrons to form positively charged ions. When an atom with less than 4. Web the marcus model forms the foundation for all modern discussion of electron transfer (et). Web ions form when atoms lose or gain electrons. When an atom loses or gains an electron, ions are formed. Web in an atom, the potential energy from moving the electron in the coulombic potential (the attraction of an. If the atom gains one or more electrons, it. Web if an atom loses or gains electrons, it will become a positively or negatively charged particle, called an ion. Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. Web solution verified by toppr correct option is a) when an atom loses an e − nuclear charge increases due to the greater number of. When an atom loses an electron, it becomes a cation (positive ion). Web thus, it is concluded that when an atom with no charge loses two electrons, it becomes a positive ion. Web (a) cation when an atom loses electrons, it becomes a positively charged ion and it is called a cation.
The Correct Option Is B Gains Or Loses Electrons.
Web in an atom, the potential energy from moving the electron in the coulombic potential (the attraction of an. When an atom gains an electron, it. Web atoms that lose electrons acquire a positive charge as a result because they are left with fewer negatively charged. Web thus, it is concluded that when an atom with no charge loses two electrons, it becomes a positive ion.
Web When Forming A Cation, An Atom Of A Main Group Element Tends To Lose All Of Its Valence Electrons, Thus Assuming The Electronic.
When an atom loses or gains an electron, ions are formed. Web solution verified by toppr correct option is a) when an atom loses an e − nuclear charge increases due to the greater number of. When an atom gains an electron it gains a. Web if an atom loses or gains electrons, it will become a positively or negatively charged particle, called an ion.
When An Atom Loses An Electron, It Becomes A Cation (Positive Ion).
Metal atoms lose electrons to form positively charged ions. Web when an atom or molecule ionizes, it either loses or gains electrons. Ions form when an atom either gains or loses electrons. An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted.
Loss Of Electrons From Atom Results In The Formation Of An Ion Known As A Cation.
When an atom with less than 4. Web study with quizlet and memorize flashcards containing terms like when an atom loses an electron, it forms a(n), the charge on a. Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. Web solution the correct option is a cation, anion cation is the name given to the positive ion of an element when it loses electrons.