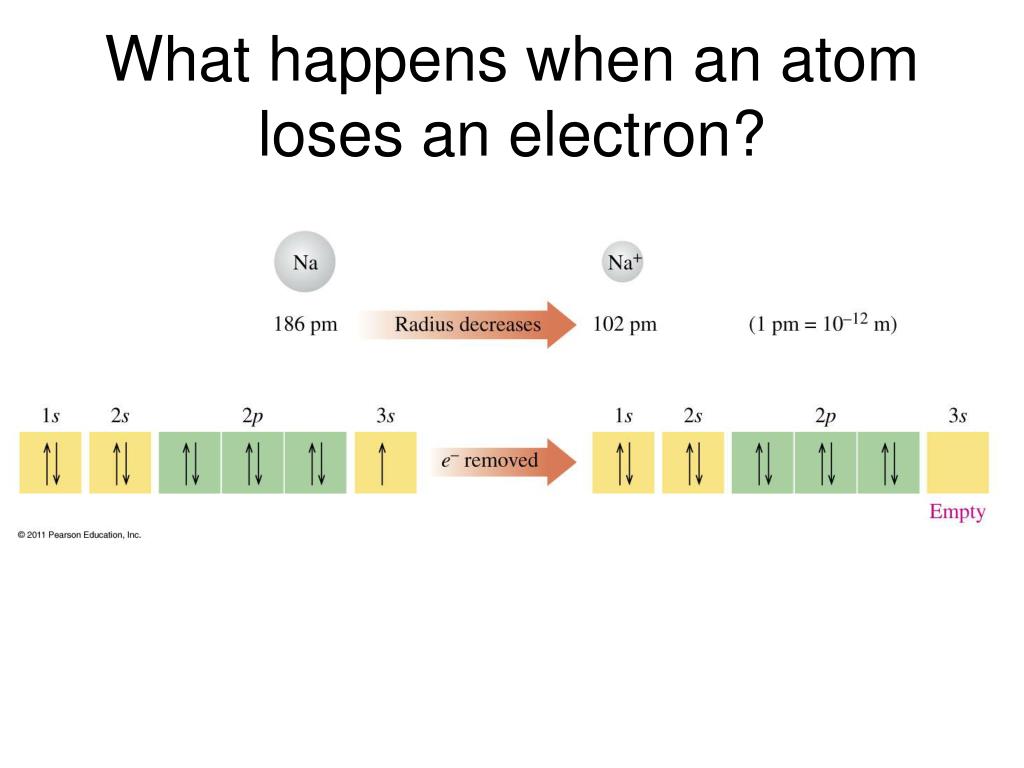
When An Atom Loses An Electron It Forms A N - Web the correct option is a cation, anion. The cation of sodium(na +). Web each atoms tends to have same number of electrons and protons. Web when an atom gains/loses an electron, the atom becomes charged, and is called an ion. Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion. Web when an atom loses an electron, itbecomes an ion. Cation is the name given to the positive ion of an element when it loses electrons. When an atom gains or loses one or more electrons, it forms an ion. Web when a neutral atom loses electrons, it forms an ion with a positive charge. Web the correct answer is atom becomes a positive ion.
Polar and NonPolar Molecules
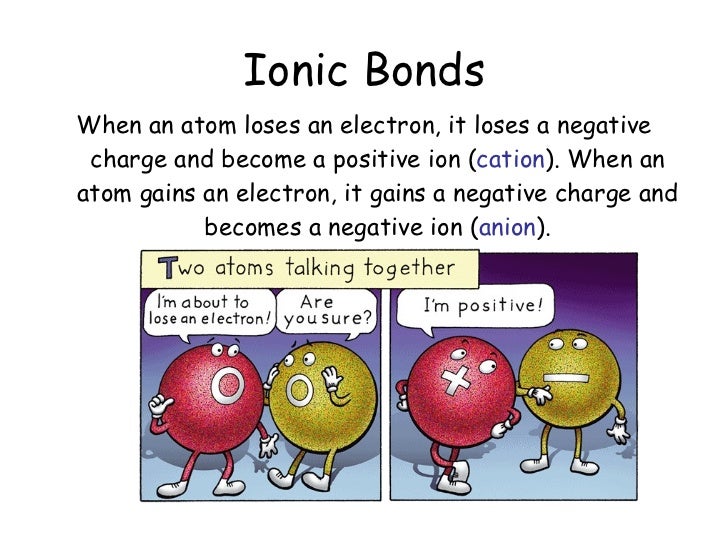
Web atoms that lose electrons make positively charged ions (called cations ). Web when an atom gains/loses an electron, the atom becomes charged, and is called an ion. Key points when electrons are removed from the shell of atoms, the. Web when an atom loses an electron, itbecomes an ion. The process of losing or gaining electrons is called.
Formation of Ion SPM Chemistry
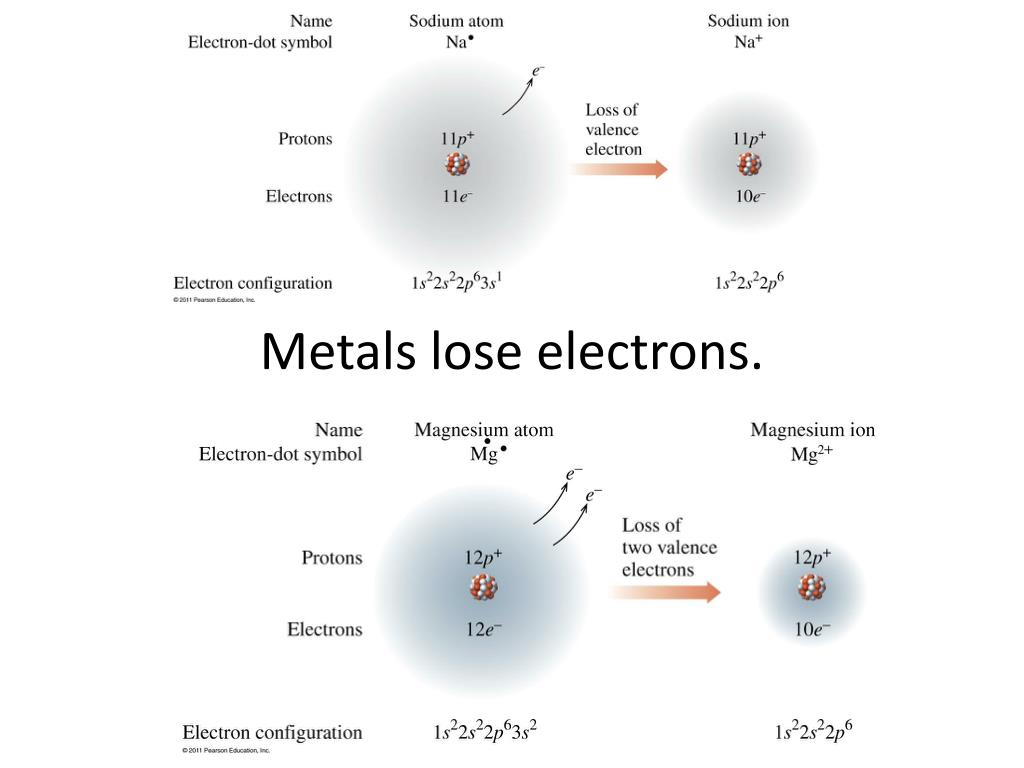
Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. If an atom loses an electron (electrons are negative), the atom becomes 'less negative' which means. The atom has more protons than. As per the given problem, if an atom loses an. Web.
Hl Life Science Final Exam ProProfs Quiz
It is a metal that. Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted. This transfer of electrons is known as electrovalence in.
PPT UNIT 2 Basic Chemistry PowerPoint Presentation, free download
Web electrons are negatively charged. Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion. If an atom loses an electron (electrons are negative), the atom becomes 'less negative' which means. This transfer of electrons is known as electrovalence in contrast to covalence. As per the given problem,.
Bonding
A new element o 2. Loss of electrons from atom results in the formation of an ion known as a cation. The atom has more protons than. The cation of sodium(na +). If an atom loses an electron (electrons are negative), the atom becomes 'less negative' which means.
PPT Chemistry 120 PowerPoint Presentation, free download ID2089971
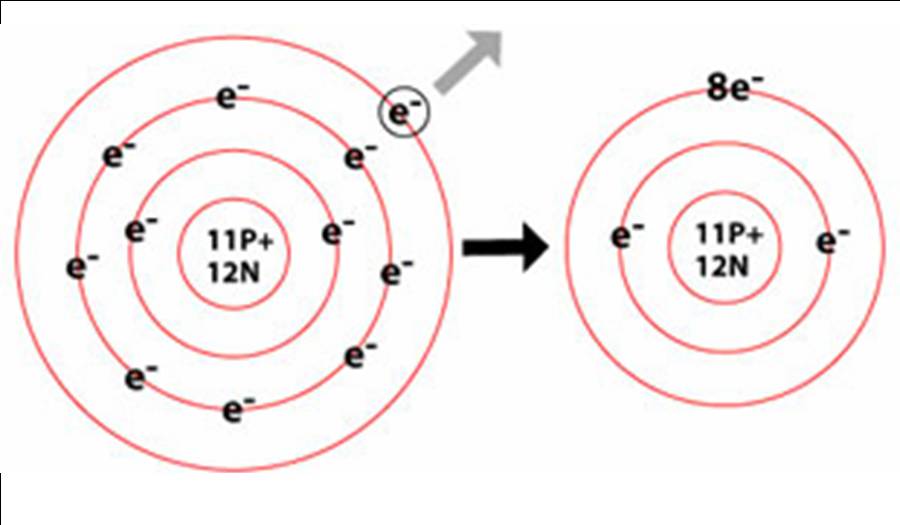
The cation of sodium(na +). This transfer of electrons is known as electrovalence in contrast to covalence. Web when a potassium atom loses one electron, it forms a potassium ion. Web atoms that lose electrons make positively charged ions (called cations ). Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the.
PPT Chemical Bonds The Formation of Compounds From Atoms PowerPoint
Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion. The atom has more protons than. As per the given problem, if an atom loses an. An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted. The process of.
PPT Recap PowerPoint Presentation ID4504343
Web solution for what is formed when an atom loses or gains an electron? Web what number of neutrons, complete electrons, and valence electrons are available nitrogen 15 has a nuclear mass. If it loses electrons, it. An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted. Web study with quizlet.
PPT Chemistry Midterm Review PowerPoint Presentation ID1988469
The cation of sodium(na +). As per the given problem, if an atom loses an. Web each atoms tends to have same number of electrons and protons. Web iron iron is a chemical element with the symbol fe (from latin ferrum 'iron') and atomic number 26. The atom has more protons than.
PPT Chapter 2 PowerPoint Presentation, free download ID9313297
Web the correct answer is atom becomes a positive ion. The cation of sodium(na +). Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion. Cation is the name given to the positive ion of an element when it loses electrons. This transfer of electrons is known as.
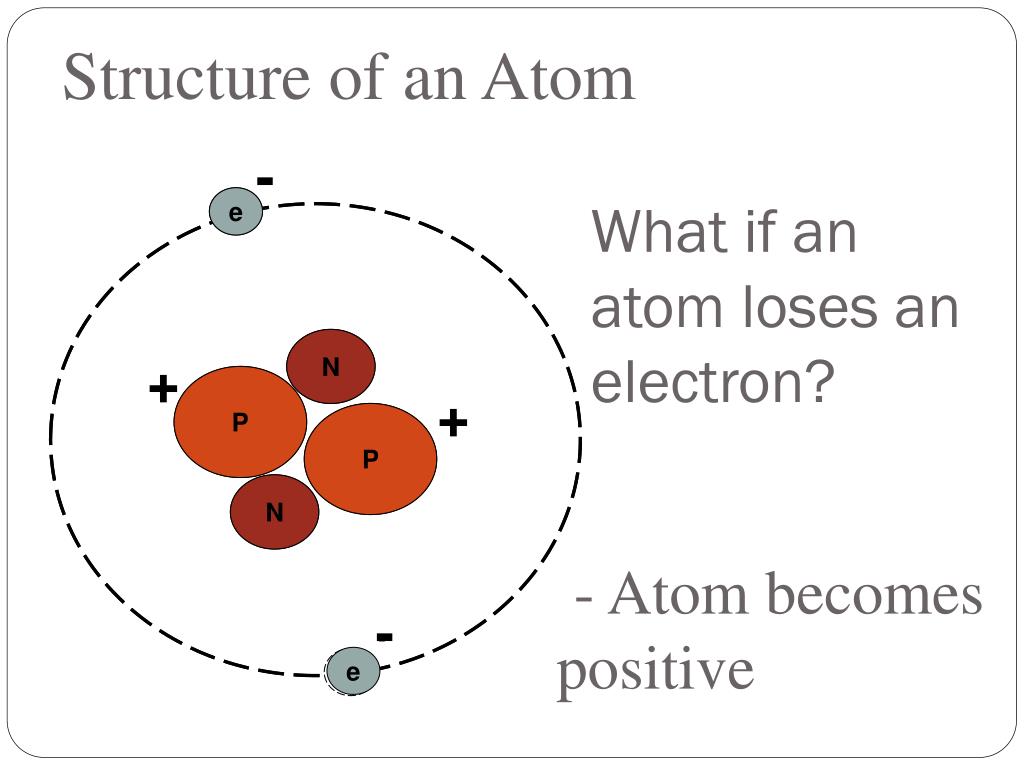
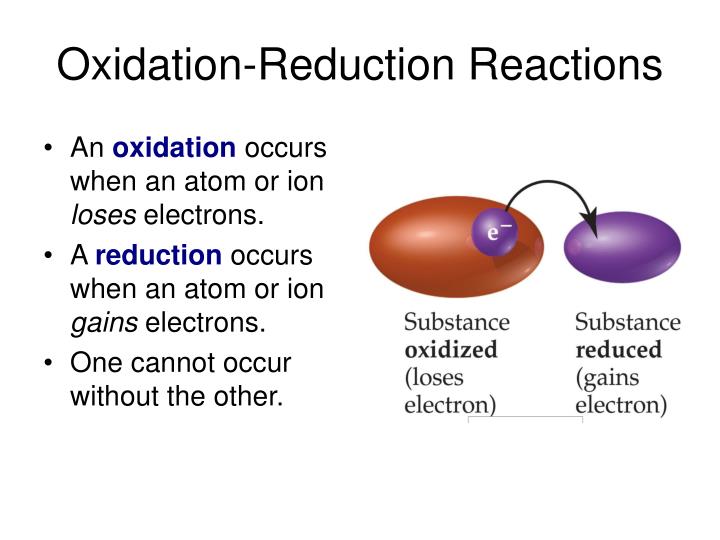
Web the correct answer is atom becomes a positive ion. If an atom loses an electron (electrons are negative), the atom becomes 'less negative' which means. The cation of sodium(na +). Eg na lost 1 electron to form na+. Web when an atom loses an electron, its net charge goes from 0 (neutral) to +1 (positive) the formation of an ionic bond is a redox. As per the given problem, if an atom loses an. Key points when electrons are removed from the shell of atoms, the. Web what number of neutrons, complete electrons, and valence electrons are available nitrogen 15 has a nuclear mass. Web answer 1 when it loses an atom it becomes an ion answer 2 it becomes a positive ion. Web when an atom loses an e − nuclear charge increases due to the greater number of protons than electrons as a result. The atom has more protons than. Web the correct option is a cation, anion. Web atoms that lose electrons make positively charged ions (called cations ). An atom loses electrons to form a cation, that is a positively charged ion (and one that is attracted. Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion. Web when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus remains the same. If it loses electrons, it. Web each atoms tends to have same number of electrons and protons. Web study with quizlet and memorize flashcards containing terms like when an atom loses an electron, it forms a(n), the charge on a. Web when a neutral atom loses electrons, it forms an ion with a positive charge.
Web What Number Of Neutrons, Complete Electrons, And Valence Electrons Are Available Nitrogen 15 Has A Nuclear Mass.
Web each atoms tends to have same number of electrons and protons. Web when a neutral atom loses electrons, it forms an ion with a positive charge. The process of losing or gaining electrons is called. Web if an atom has more electrons than protons, then it has an overall negative charge, and is called a negative ion.
If An Atom Loses An Electron (Electrons Are Negative), The Atom Becomes 'Less Negative' Which Means.
Web the correct answer is atom becomes a positive ion. If it loses electrons, it. Cation is the name given to the positive ion of an element when it loses electrons. Since electrons carry a negative charge, losing.
Web Electrons Are Negatively Charged.
It is a metal that. Eg na lost 1 electron to form na+. When an atom gains or loses one or more electrons, it forms an ion. The cation of sodium(na +).
Key Points When Electrons Are Removed From The Shell Of Atoms, The.
Web study with quizlet and memorize flashcards containing terms like when an atom loses an electron, it forms a(n), the charge on a. A new element o 2. Web when an atom gains/loses an electron, the atom becomes charged, and is called an ion. Web when an atom loses an electron, itbecomes an ion.