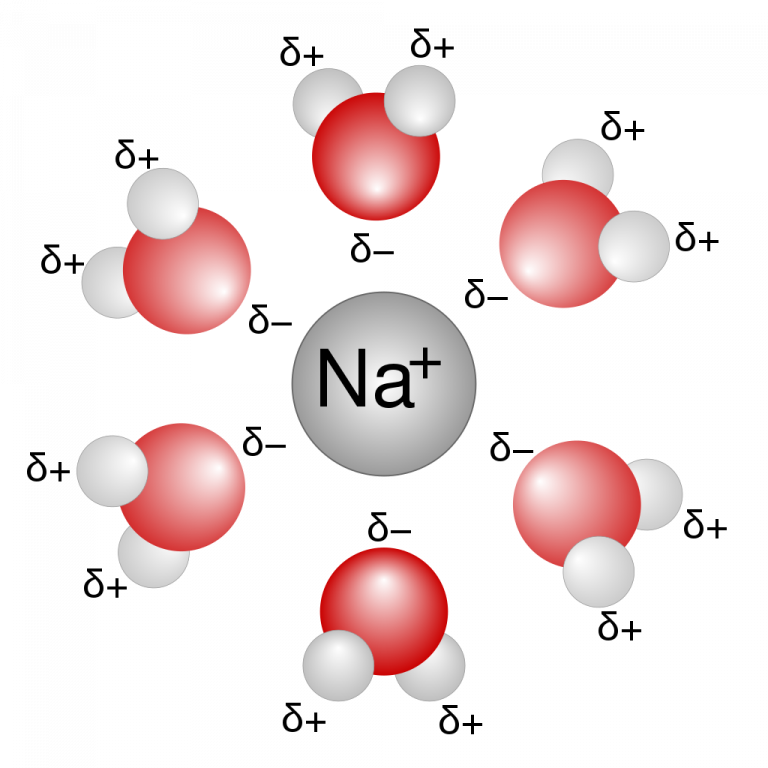
What Structures Are Formed When Water Molecules Surrounds Individual Ions - Web in simple ionic structures, the water molecules surround themselves with the oppositely charged ion. Web when the substance is placed in water, the water molecules pull the positive and negative ions apart from each other. Web ionic bonds are generally formed when electrons are transferred from one atom to another, causing individual. Web in the case of table salt (nacl) mixed in water (figure 3), the sodium and chloride ions separate, or dissociate, in the water, and. In water molecules, due to bonding. Web this process, in which either a positive or a negative ion attracts water molecules to its immediate vicinity, is called. Web these cooperative structural changes of water molecules around an ion cause the breaking of the bridging h. Web liquid and solid water. Web hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres. Web cohesion when water molecules stick to other polar substances.
Chemical Bonds · Anatomy and Physiology
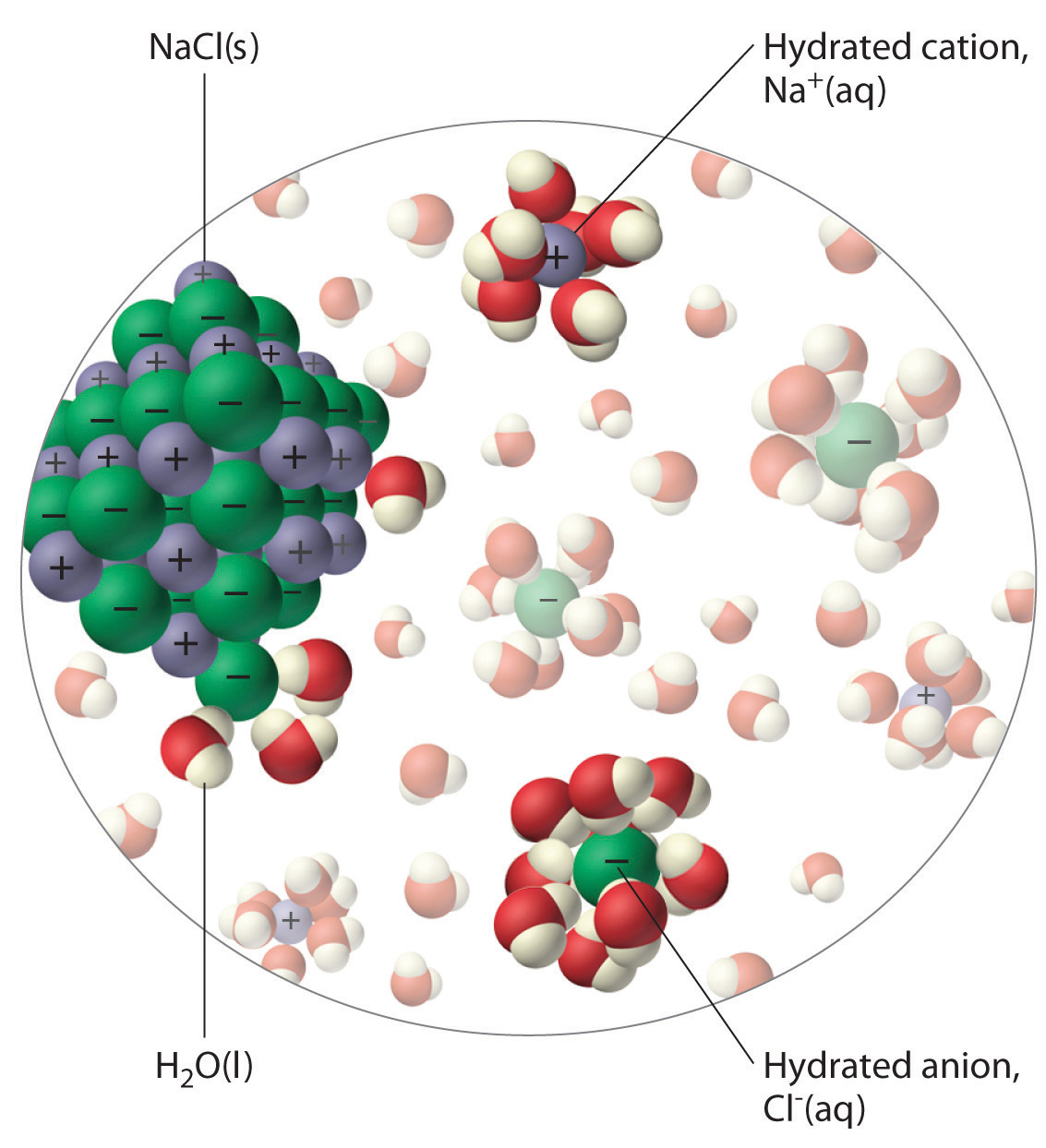
Web the water molecules affect the formation of the ion pair. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the anions (negatively. Web cohesion when water molecules stick to other polar substances. Web but the attractions between these ions and water molecules is greater than their attractions for each other. Web liquid and.
Liquid Properties Boundless Chemistry
Web the major constituent of the human body (over \ (60\%\)) is water. Web small ions (kosmotropes) have high charge densities so they cause strong electrostatic ordering of nearby waters,. Web what structures are formed when water molecules surrounds individual ions? Web when the substance is placed in water, the water molecules pull the positive and negative ions apart from.
Assalamualaikum....
Web cohesion when water molecules stick to other polar substances. It also depicts how a charge,. They can either form a joint hydration shell around the two. Web water is a molecule that has the ability to attract ions and form new chemical compounds called hydrates. Web what structures are formed when water molecules surrounds individual ions?
Aqueous Solutions
Learn about the basic structure of an ion, related to atomic number and mass. Web liquid and solid water. Web in simple ionic structures, the water molecules surround themselves with the oppositely charged ion. This simple molecule plays important roles in all kinds. Web water is a molecule that has the ability to attract ions and form new chemical compounds.
Nature up close Water molecules CBS News
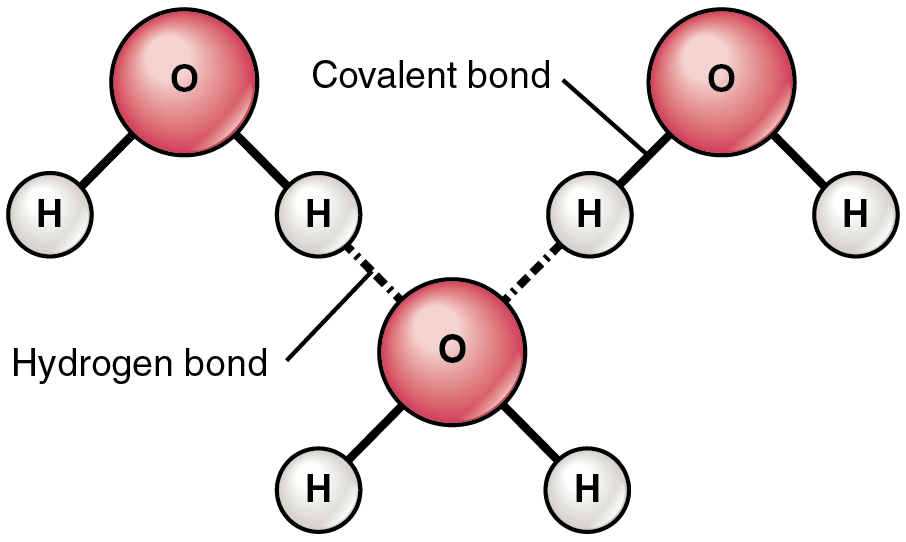
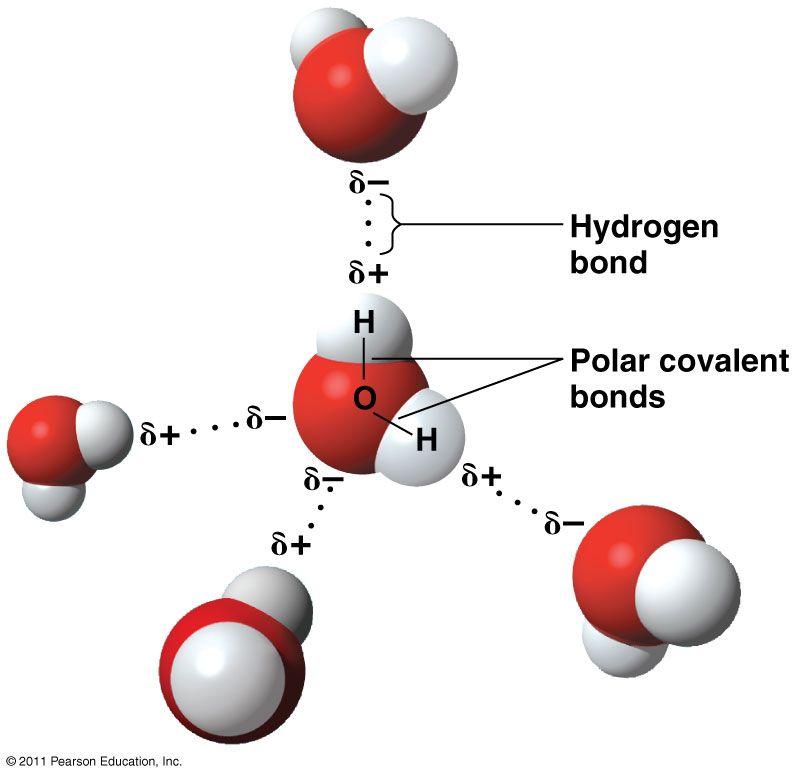
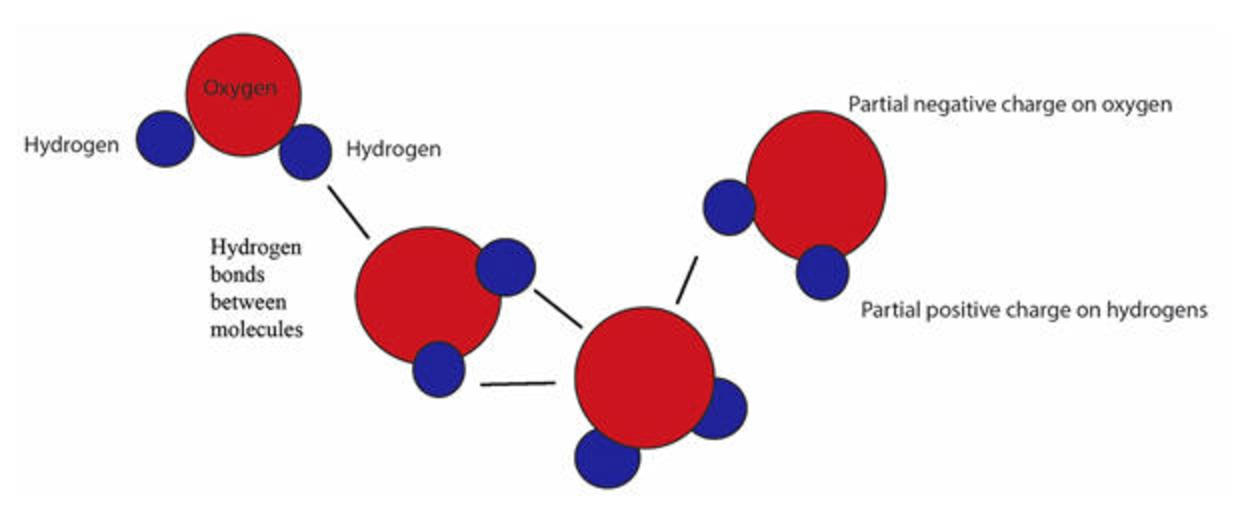
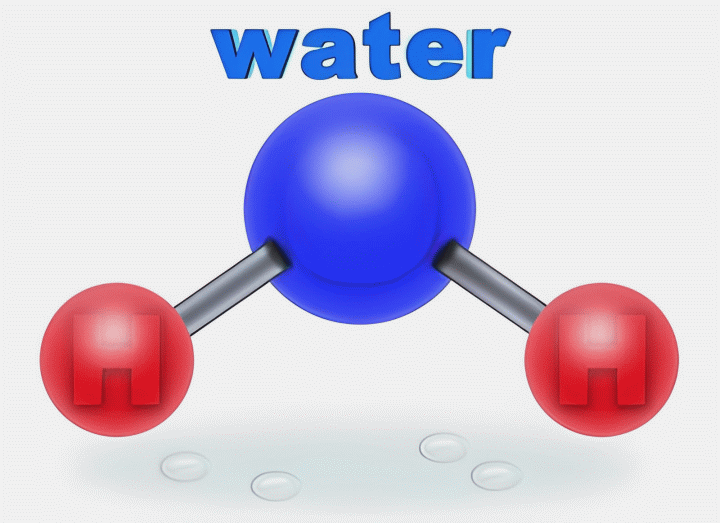
Web what structures are formed when water molecules surrounds individual ions? In water molecules, due to bonding. This simple molecule plays important roles in all kinds. Adhesion molecules with partial charges on opposite ends are. Web this diagram shows the positive and negative parts of a water molecule.
Fundamentals of Physics and Chemistry Important to Microbiology
Learn about the basic structure of an ion, related to atomic number and mass. It also depicts how a charge,. Web the major constituent of the human body (over \ (60\%\)) is water. Web hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres. Web in simple ionic structures, the water molecules surround themselves with.
Water
It also depicts how a charge,. Chemistry matter phases of matter 1 answer anor277 may 22,. Web the major constituent of the human body (over \ (60\%\)) is water. This simple molecule plays important roles in all kinds. Learn about the basic structure of an ion, related to atomic number and mass.
chemistry Dissociation of HCl in aqueous solution
Web small ions (kosmotropes) have high charge densities so they cause strong electrostatic ordering of nearby waters,. Web cohesion when water molecules stick to other polar substances. Web this diagram shows the positive and negative parts of a water molecule. Web these cooperative structural changes of water molecules around an ion cause the breaking of the bridging h. Each water.
Solubility Rules The Ultimate Guide to AP® Chemistry Albert.io
Web hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres. Web ionic bonds are generally formed when electrons are transferred from one atom to another, causing individual. Web in simple ionic structures, the water molecules surround themselves with the oppositely charged ion. Web the water molecules affect the formation of the ion pair. Web.
Ion producing appliances and cosmetology Understand ions
Each water molecule is surrounded by four. Web water is a molecule that has the ability to attract ions and form new chemical compounds called hydrates. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the anions (negatively. Web when the substance is placed in water, the water molecules pull the positive and negative.
Web when the substance is placed in water, the water molecules pull the positive and negative ions apart from each other. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the. It also depicts how a charge,. Web in the case of table salt (nacl) mixed in water (figure 3), the sodium and chloride ions separate, or dissociate, in the water, and. Web small ions (kosmotropes) have high charge densities so they cause strong electrostatic ordering of nearby waters,. Web in simple ionic structures, the water molecules surround themselves with the oppositely charged ion. Web cohesion when water molecules stick to other polar substances. Web ionic bonds are generally formed when electrons are transferred from one atom to another, causing individual. They can either form a joint hydration shell around the two. Web liquid and solid water. Adhesion molecules with partial charges on opposite ends are. Web water is a molecule that has the ability to attract ions and form new chemical compounds called hydrates. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the anions (negatively. Web the water molecules affect the formation of the ion pair. Web when electrons are transferred and ions form, ionic bonds result. Web these cooperative structural changes of water molecules around an ion cause the breaking of the bridging h. Each water molecule is surrounded by four. Chemistry matter phases of matter 1 answer anor277 may 22,. Web the major constituent of the human body (over \ (60\%\)) is water. Web this diagram shows the positive and negative parts of a water molecule.
Web What Structures Are Formed When Water Molecules Surrounds Individual Ions?
This simple molecule plays important roles in all kinds. Learn about the basic structure of an ion, related to atomic number and mass. Web the water molecules affect the formation of the ion pair. Web this process, in which either a positive or a negative ion attracts water molecules to its immediate vicinity, is called.
Web In The Case Of Table Salt (Nacl) Mixed In Water (Figure 3), The Sodium And Chloride Ions Separate, Or Dissociate, In The Water, And.
Web when the substance is placed in water, the water molecules pull the positive and negative ions apart from each other. Web but the attractions between these ions and water molecules is greater than their attractions for each other. Web this diagram shows the positive and negative parts of a water molecule. Web ionic bonds are generally formed when electrons are transferred from one atom to another, causing individual.
Web These Cooperative Structural Changes Of Water Molecules Around An Ion Cause The Breaking Of The Bridging H.
Web liquid and solid water. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the anions (negatively. Web water is a molecule that has the ability to attract ions and form new chemical compounds called hydrates. Web cohesion when water molecules stick to other polar substances.
They Can Either Form A Joint Hydration Shell Around The Two.
Web hence, structures formed when a water molecule surrounds individual ions are known as hydration spheres. Cations (positively charged ions) are surrounded by the negative dipole of the water molecule and the. Web in simple ionic structures, the water molecules surround themselves with the oppositely charged ion. Chemistry matter phases of matter 1 answer anor277 may 22,.