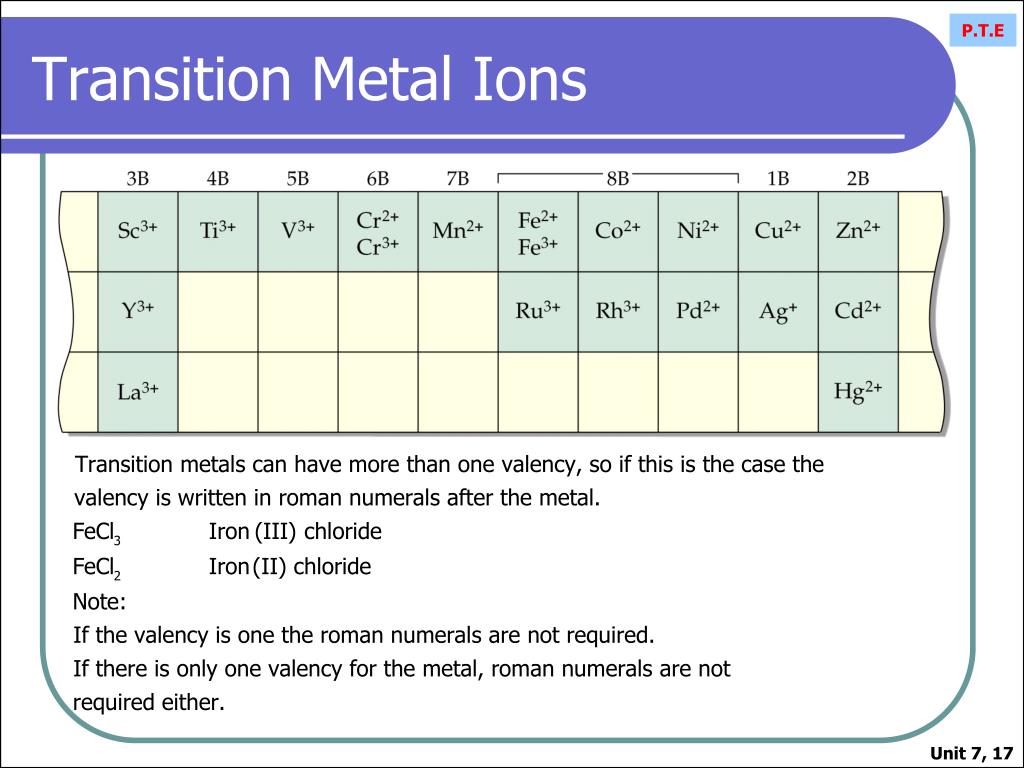
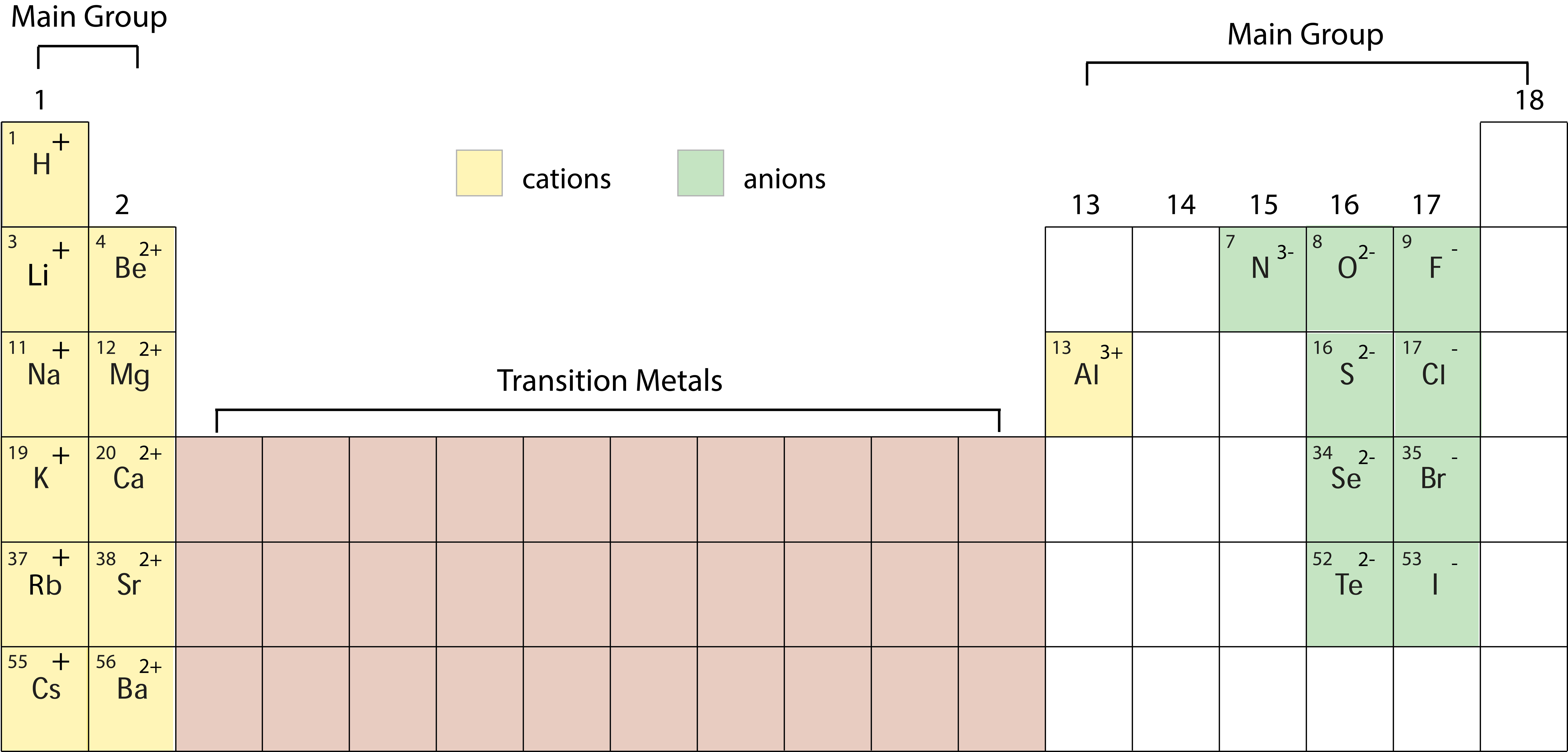
What Kind Of Ions Do Metals Form - Web chemistry library unit 1: Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Web summary transition metals have unfilled inner d electron shells. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and. Web metals usually form cations while nonmetals usially form anions. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web best answer copy metals tend to form cations (positive ions). They do this because they need to. And thus metals tend to form positive ions. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2 + is called a calcium ion.
PPT Chapter 4 Compounds and Their Bonds PowerPoint Presentation, free
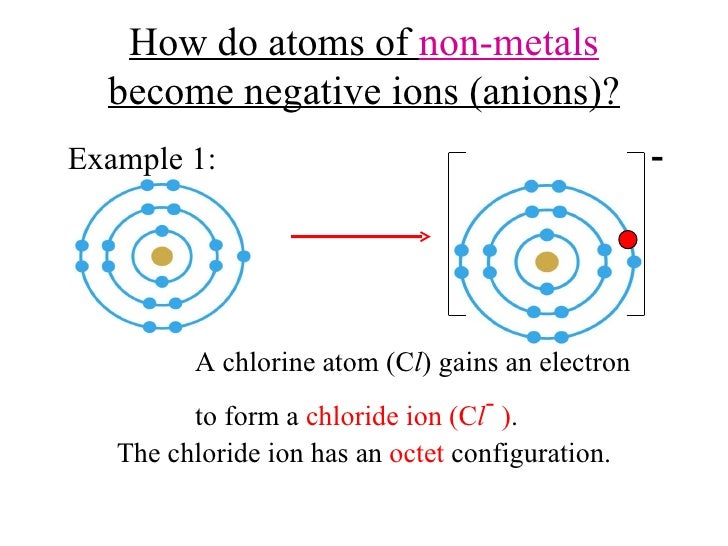
Nonmetals form negatively charged ions, or anions. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or. They do this because they need to. Web metal atoms lose electrons from their outer shell when they form ions: One way to predict the type of bond that forms between two elements is.
Ionic Bond Definition, Types, Properties & Examples
The ions are positive, because they have more protons. And thus metals tend to form positive ions. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Chemwiki ucdavis] since it is written that metals usually. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen.
PPT Atomic Theory PowerPoint Presentation, free download ID4961184
Web best answer copy metals tend to form cations (positive ions). The ions are positive, because they have more protons. The scientific name for positively charged ions. M + δ → m 2+ + 2e−. Ions form primarily through loss of s.
Chem matters ch6_ionic_bond
Web metals usually form cations while nonmetals usially form anions. Web summary transition metals have unfilled inner d electron shells. Atoms that gain electrons make negatively charged ions (called anions ). Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. Web ions consisting of only.
Periodic Table Ions List Periodic Table Timeline
Web ions are atoms (or groups of atoms) with an electrostatic charge. The scientific name for positively charged ions. Metal atoms become positively charged ions. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or. At an atomic level, the valence.
The Temptation News periodic table of elements with charges of ions
Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. The scientific name for positively charged ions. Nonmetals form negatively charged ions, or anions. Web summary transition metals have unfilled inner d electron shells.
Chem matters ch6_ionic_bond
Web the type of ions that metals form are called positively charged ions. M + δ → m 2+ + 2e−. Metal atoms become positively charged ions. At an atomic level, the valence. Web summary transition metals have unfilled inner d electron shells.
Chem Ions Scientific Tutor
Chemwiki ucdavis] since it is written that metals usually. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Lesson 3 names and formulas of ionic compounds naming monatomic ions and ionic compounds common. Atoms that gain electrons make negatively charged ions (called anions ). Web ion, any atom or group of atoms that bears one.
PreChemistry
Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and. The scientific name for positively charged ions. Web metal atoms lose electrons from their outer shell when they form ions: According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or. M + δ.
Chem matters ch6_ionic_bond
The ions are positive, because they have more protons. Lesson 3 names and formulas of ionic compounds naming monatomic ions and ionic compounds common. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web chemistry library unit 1: At an atomic level, the valence.
Web octahedral complexes containing period 5 or 6 metal centers, or high oxidation numbers, are less. Web chemistry library unit 1: Atoms that gain electrons make negatively charged ions (called anions ). Web video test 1 2 3 4 forming ions an ion is an atom or group of atoms with a positive or negative charge. And thus metals tend to form positive ions. Web metal atoms lose electrons from their outer shell when they form ions: Web ions consisting of only a single atom are termed atomic or monatomic ions, while two or more atoms form molecular ions or. The ions are positive, because they have more protons. At an atomic level, the valence. Web transition metal ions are essential cofactors for proteins with diverse functions, including electron transfer, dioxygen binding and. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web group iv a metals form cations with a +4 charge, whereas tin (sn) and lead (pb) can form cations with a +2 charge. Web the type of ions that metals form are called positively charged ions. Web ions are atoms (or groups of atoms) with an electrostatic charge. According to the spdf theory, metals can have either a fixed number of electrons that they can lose, or. Web summary transition metals have unfilled inner d electron shells. Metal atoms become positively charged ions. Nonmetals form negatively charged ions, or anions. Web best answer copy metals tend to form cations (positive ions). One way to predict the type of bond that forms between two elements is to consider whether each element is a.
Web The Type Of Ions That Metals Form Are Called Positively Charged Ions.
Web metals form positive ions and nonmetals negative ions. Web the name of a metal ion is the same as the name of the metal atom from which it forms, so ca 2 + is called a calcium ion. Metal atoms become positively charged ions. Chemwiki ucdavis] since it is written that metals usually.
Web Metal Atoms Lose Electrons From Their Outer Shell When They Form Ions:
Web summary transition metals have unfilled inner d electron shells. Nonmetals form negatively charged ions, or anions. M + δ → m 2+ + 2e−. Ions form primarily through loss of s.
Web Transition Metal Ions Are Essential Cofactors For Proteins With Diverse Functions, Including Electron Transfer, Dioxygen Binding And.
At an atomic level, the valence. Web ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Web metals usually form cations while nonmetals usially form anions. One way to predict the type of bond that forms between two elements is to consider whether each element is a.
Metals Are Defined As The Elements Which Loose Electrons To Attain Stable Electronic.
The ions are positive, because they have more protons. Web ions are atoms (or groups of atoms) with an electrostatic charge. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web octahedral complexes containing period 5 or 6 metal centers, or high oxidation numbers, are less.