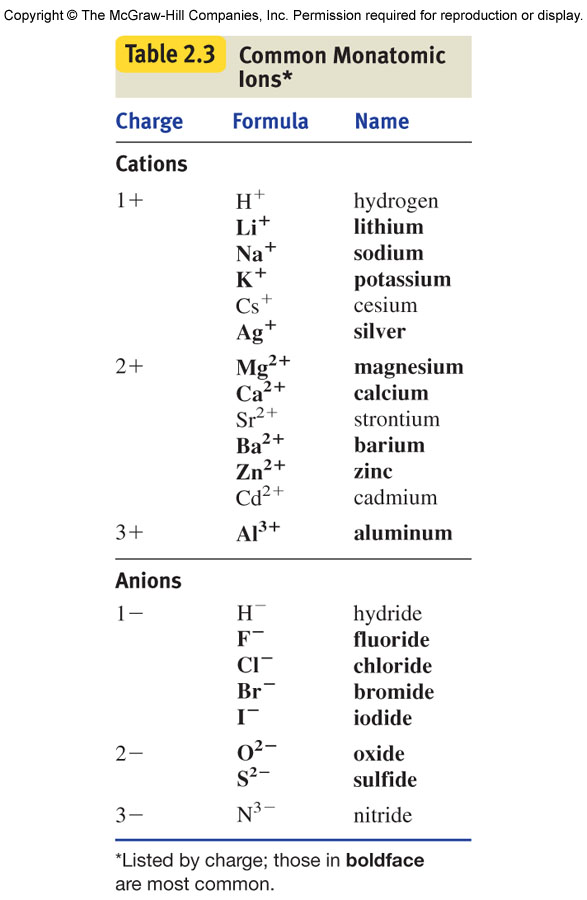
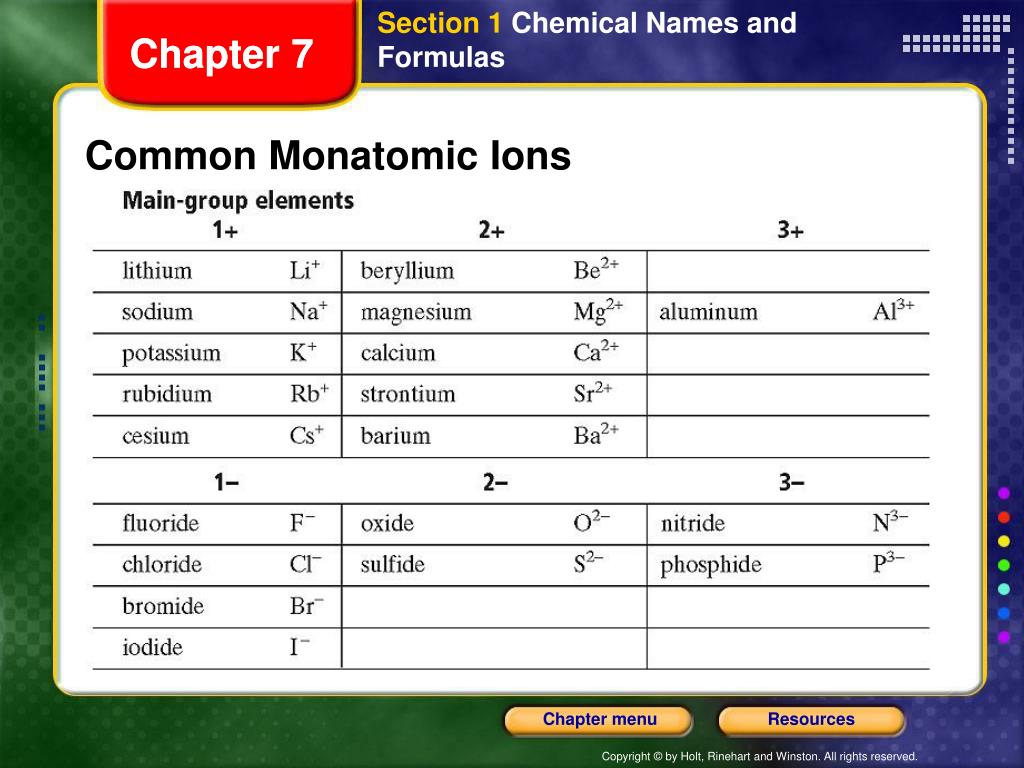
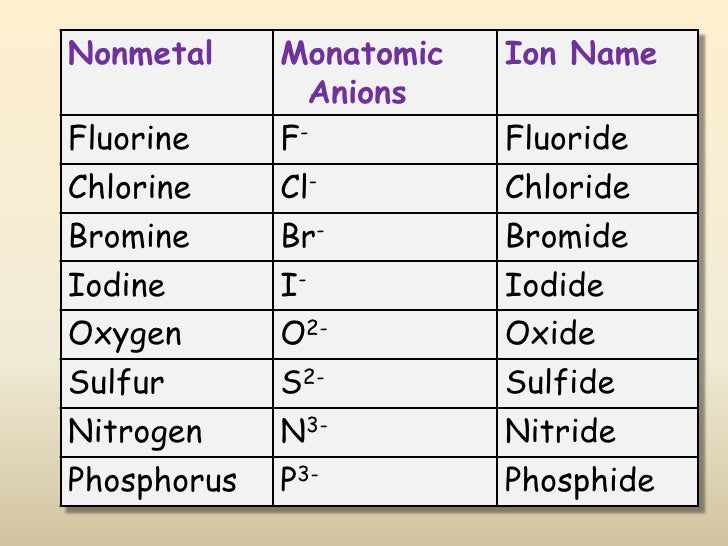
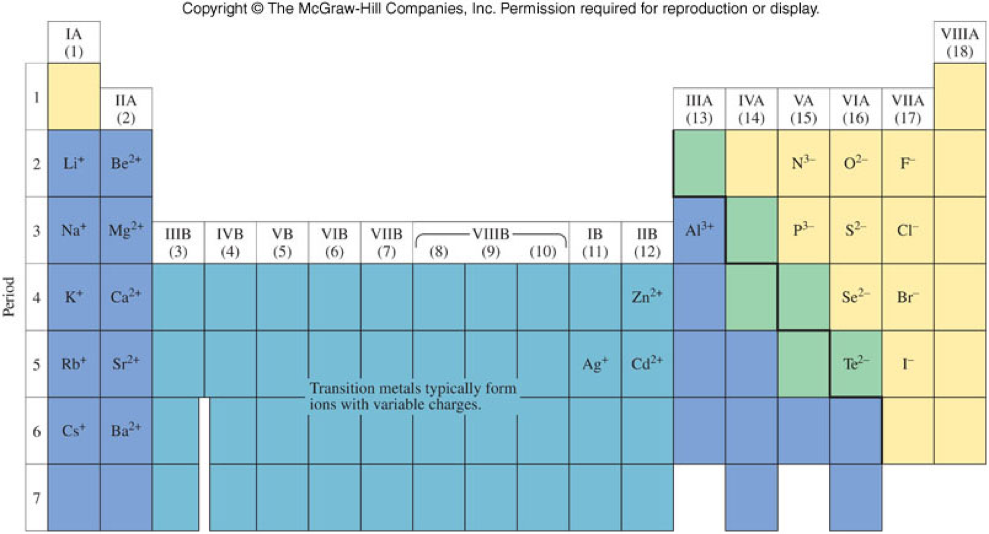
What Is The Most Stable Monatomic Ion Formed From Nitrogen - Web technically, a monatomic ion is a form of monatomic atom. University professor with 10+ years tutoring experience. Emeraldchest99 is waiting for your help. Web because these ions contain more than one atom, they are called polyatomic ions. The meaning becomes clear if taken as three parts. In this article, we will discuss polyatomic ions. Web what is the most stable monatomic ion formed from nitrogen. However, the term monatomic atom usually refers to. Web the term monatomic ion sounds complex. The most stable monoatomic ion formed will be explanation:
PPT Chapter 7 Chemical Formulas and Chemical Compounds PowerPoint
Emeraldchest99 is waiting for your help. Web the term monatomic ion sounds complex. Web nonmetals form negative ions (anions). In this article, we will discuss polyatomic ions. Web the only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (stp) (or typical.
UWEau Claire, Chem 103, Section F0F
For example, fluorine will want 1 more electron to fill it's. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. Web the symbol for the ion is mg 2+, and it is called a magnesium ion. The ammonium ion ( nh 4 +) is a nitrogen atom (blue) bonded to four.
Monatomic Ion Easy Science Easy science, Ap chemistry, Chemistry
Web nonmetals form negative ions (anions). Web the most stable monatomic ion will occur when the atom's electron shells are all completely full. Monoatomic ion is defined as. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. The most stable monoatomic ion formed will be explanation:
PPT Chemical Names and Formulas PowerPoint Presentation, free
Web technically, a monatomic ion is a form of monatomic atom. Web the term monatomic ion sounds complex. A nitrogen atom must gain three electrons to have the same number of electrons as an. The meaning becomes clear if taken as three parts. Web what is the most stable monatomic ion formed from nitrogen?
PPT Monatomic Ions PowerPoint Presentation, free download ID6687141
The most stable monoatomic ion formed will be explanation: Web the symbol for the ion is mg 2+, and it is called a magnesium ion. Web the only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (stp) (or typical. Web what is the most stable monatomic ion formed from nitrogen? Web write the lewis symbols.
PPT Chapter 6 PowerPoint Presentation, free download ID3545809
Web what is the most stable monatomic ion formed from fluorine? The ammonium ion ( nh 4 +) is a nitrogen atom (blue) bonded to four hydrogen atoms (white). Web the only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (stp) (or typical. Nitrogen’s position in the periodic table (group 15) reveals that it is.
Ionic bonding
Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. In this article, we will discuss polyatomic ions. Web technically, a monatomic ion is a form of monatomic atom. Web a monatomic ion (also called simple ion) is an ion consisting of exactly one atom. Web the term monatomic ion sounds complex.
PPT Chapter 2 Molecules, Ions, and Compounds PowerPoint Presentation
Web the term monatomic ion sounds complex. Web what is the most stable monatomic ion formed from fluorine? A nitrogen atom must gain three electrons to have the same number of electrons as an. Emeraldchest99 is waiting for your help. Web a monatomic ion (also called simple ion) is an ion consisting of exactly one atom.
Chapter 3 Presentation
Web what is the most stable monatomic ion formed from nitrogen. Web the most stable monatomic ion will occur when the atom's electron shells are all completely full. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Web write the lewis symbols for the monatomic ions formed from the following elements: Emeraldchest99 is waiting for.
Ionic Naming
Web what is the most stable monatomic ion formed from nitrogen? However, the term monatomic atom usually refers to. The meaning becomes clear if taken as three parts. Web technically, a monatomic ion is a form of monatomic atom. Because fluorine has 7 valence.
Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. However, the term monatomic atom usually refers to. Web write the lewis symbols for the monatomic ions formed from the following elements: Web nonmetals form negative ions (anions). Web what is the most stable monatomic ion formed from fluorine? Because fluorine has 7 valence. The ammonium ion ( nh 4 +) is a nitrogen atom (blue) bonded to four hydrogen atoms (white). Web what is the most stable monatomic ion formed from nitrogen? Web because these ions contain more than one atom, they are called polyatomic ions. Nitrogen’s position in the periodic table (group 15) reveals that it is a nonmetal. Web there is a certain stability within an atom when the electrons have a full valence shell (8 electrons in the outer. In this article, we will discuss polyatomic ions. For example, fluorine will want 1 more electron to fill it's. The meaning becomes clear if taken as three parts. Monoatomic ion is defined as. Web technically, a monatomic ion is a form of monatomic atom. Web to obtain noble gas configuration, it only has 1 less electron, so it gains 1 electron, completes its valence shell, and achieves. Web what is the most stable monatomic ion formed from nitrogen. Web the only chemical elements that form stable homonuclear diatomic molecules at standard temperature and pressure (stp) (or typical. Web a monatomic ion (also called simple ion) is an ion consisting of exactly one atom.
Monoatomic Ion Is Defined As.
The most stable monoatomic ion formed will be explanation: Web because these ions contain more than one atom, they are called polyatomic ions. Web the symbol for the ion is mg 2+, and it is called a magnesium ion. Web there is a certain stability within an atom when the electrons have a full valence shell (8 electrons in the outer.
Web The Most Stable Monatomic Ion Will Occur When The Atom's Electron Shells Are All Completely Full.
Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. Web what is the most stable monatomic ion formed from nitrogen? Emeraldchest99 is waiting for your help. However, the term monatomic atom usually refers to.
The Ammonium Ion ( Nh 4 +) Is A Nitrogen Atom (Blue) Bonded To Four Hydrogen Atoms (White).
Web technically, a monatomic ion is a form of monatomic atom. Web nonmetals form negative ions (anions). Because fluorine has 7 valence. University professor with 10+ years tutoring experience.
Web The Only Chemical Elements That Form Stable Homonuclear Diatomic Molecules At Standard Temperature And Pressure (Stp) (Or Typical.
A nitrogen atom must gain three electrons to have the same number of electrons as an. Web the term monatomic ion sounds complex. For example, fluorine will want 1 more electron to fill it's. (a) cl (b) na (c) mg (d) ca (e) k (f) br (g) sr (h).