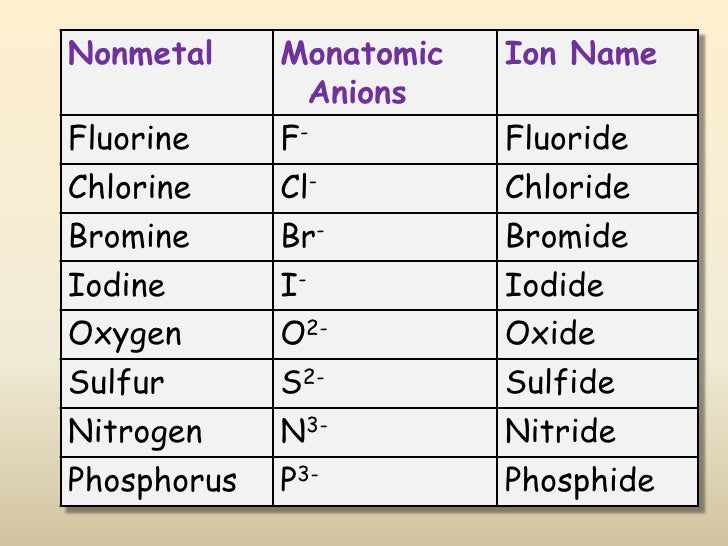
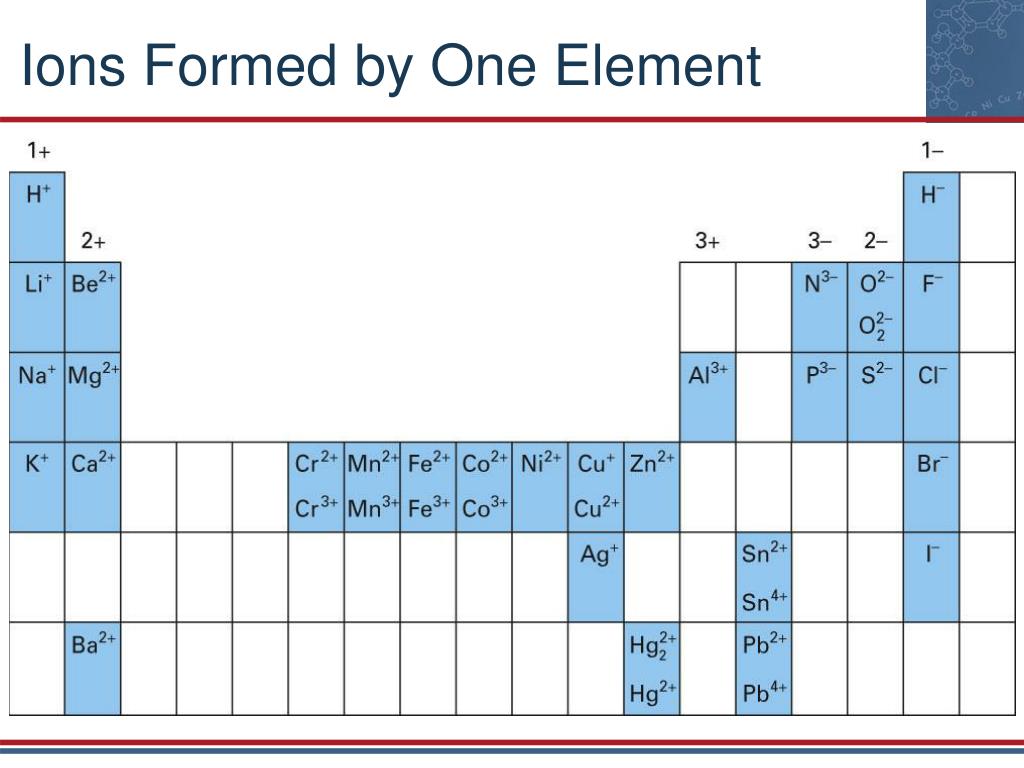
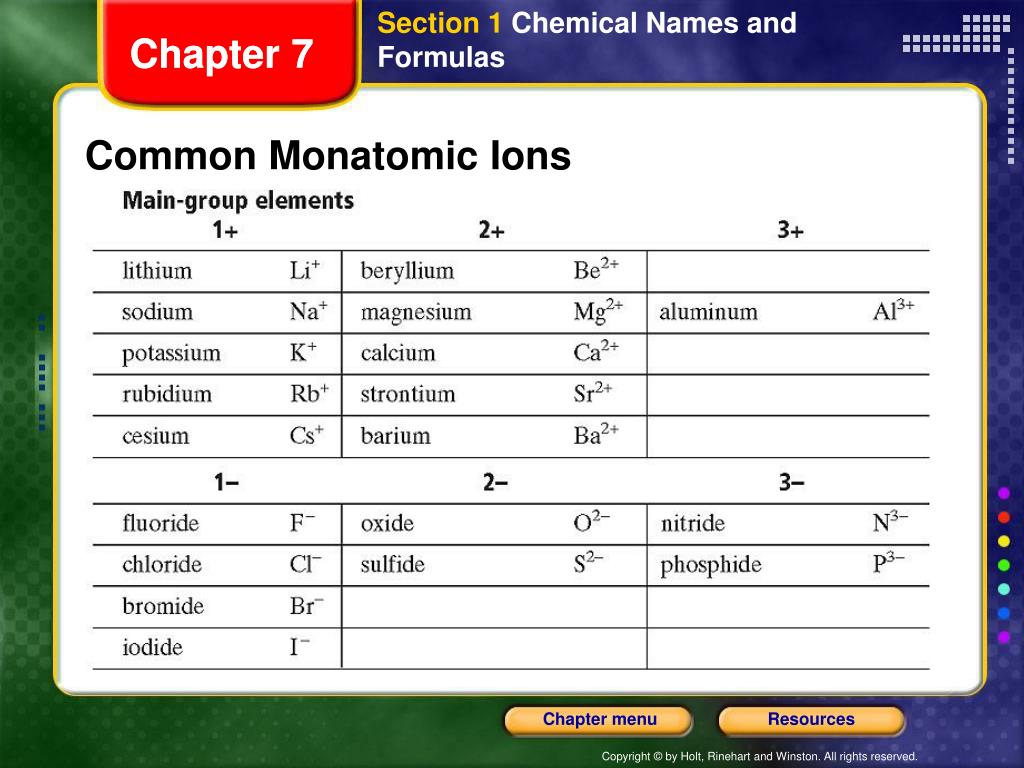
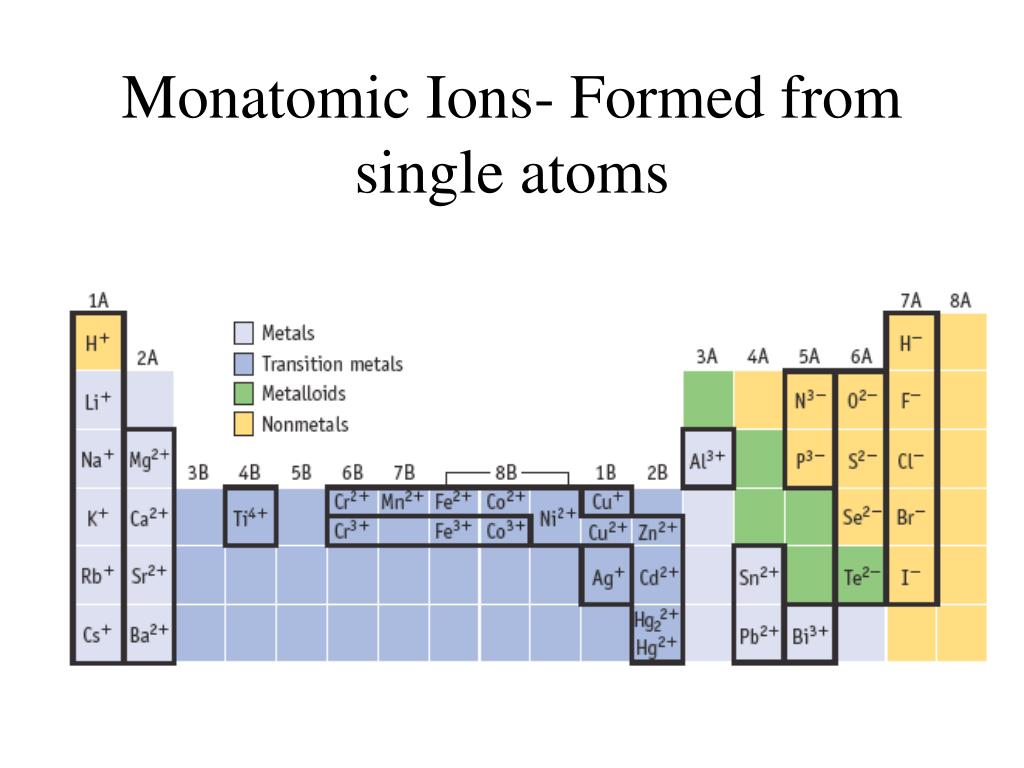
What Is The Most Stable Monatomic Ion Formed From Fluorine - Ions are formed when an atom looses or. Though believed not to occur naturally,. The ion formed by lithium element is explanation: Web forming an octet (eight electrons in the outer shell) provides stability to the atom. A monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. Neutral atom of fluorine has 7 valence electrons. Web about this tutor ›. Web fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent. Fluorine will gain one electron.
Ionic bonding
Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web about this tutor ›. It has one electron more than its atom, so te electron occupies the last p orbital, fills the p shell. Web this property makes organofluorine compounds some of the most stable and inert substances known to man. Web what is the most.
PPT Chapter 2 Atoms, Molecules, and Ions PowerPoint Presentation
Web the most stable monatomic ion will occur when the atom's electron. Though believed not to occur naturally,. Web what is the most stable monatomic ion formed from fluorine? Web fluorine is a lewis acid in weak acid, which means that it accepts. Fluorine will gain one electron.
PPT IONIC COMPOUNDS PowerPoint Presentation, free download ID4686657
The stable mono atomic ion of fluorine is. Web this property makes organofluorine compounds some of the most stable and inert substances known to man. Web fluorine is a lewis acid in weak acid, which means that it accepts. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Mono atomic ion is.
PPT Ionic Compounds and Metals PowerPoint Presentation, free download
Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen). Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web fluorine is a group 7a element. Web fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent. Web fluorine is a.
PPT Chapter 6 Chemical Nomenclature PowerPoint Presentation, free
Web as the most electronegative element, fluorine also cannot be a central atom. Most frequently, covalent bonds involving. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen). Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. It has one electron more than its atom, so te electron occupies the last p orbital,.
PPT Chemical Names and Formulas PowerPoint Presentation, free
Web the most stable monatomic ion will occur when the atom's electron. It has one electron more than its atom, so te electron occupies the last p orbital, fills the p shell. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. Neutral atom of fluorine has 7 valence electrons. A monatomic.
PPT Chapter 6 PowerPoint Presentation, free download ID3545809
Web fluorine is a group 7a element. Web fluorine is a lewis acid in weak acid, which means that it accepts. Fluorine will gain one electron. Web forming an octet (eight electrons in the outer shell) provides stability to the atom. Neutral atom of fluorine has 7 valence electrons.
Ionic Naming
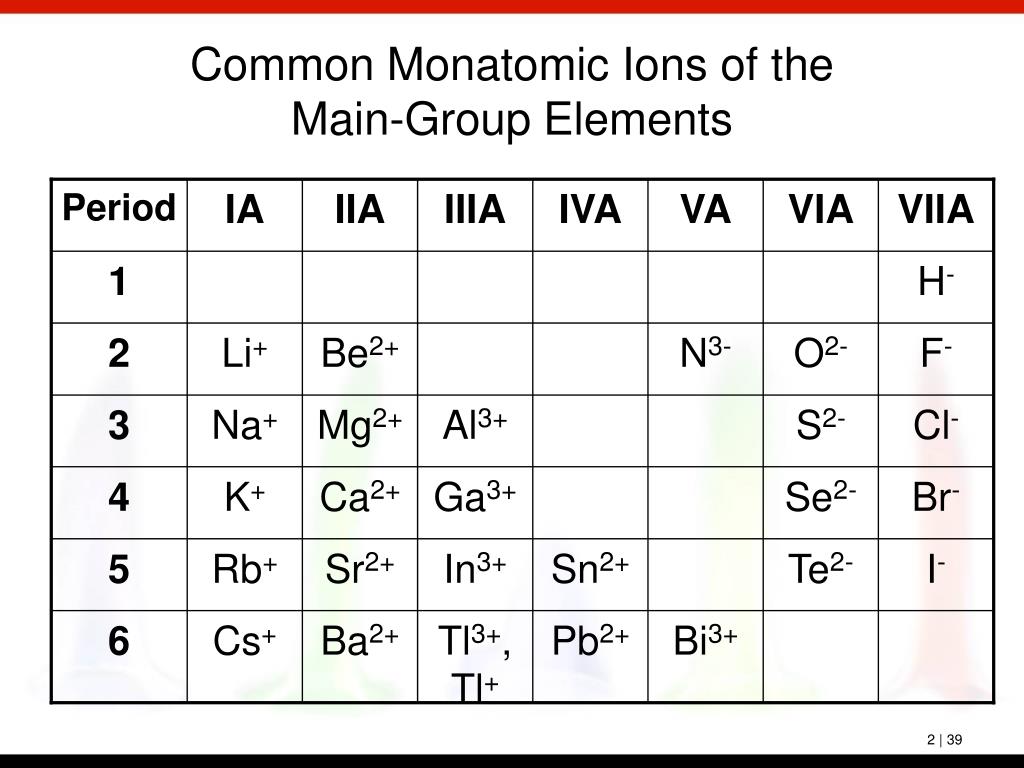
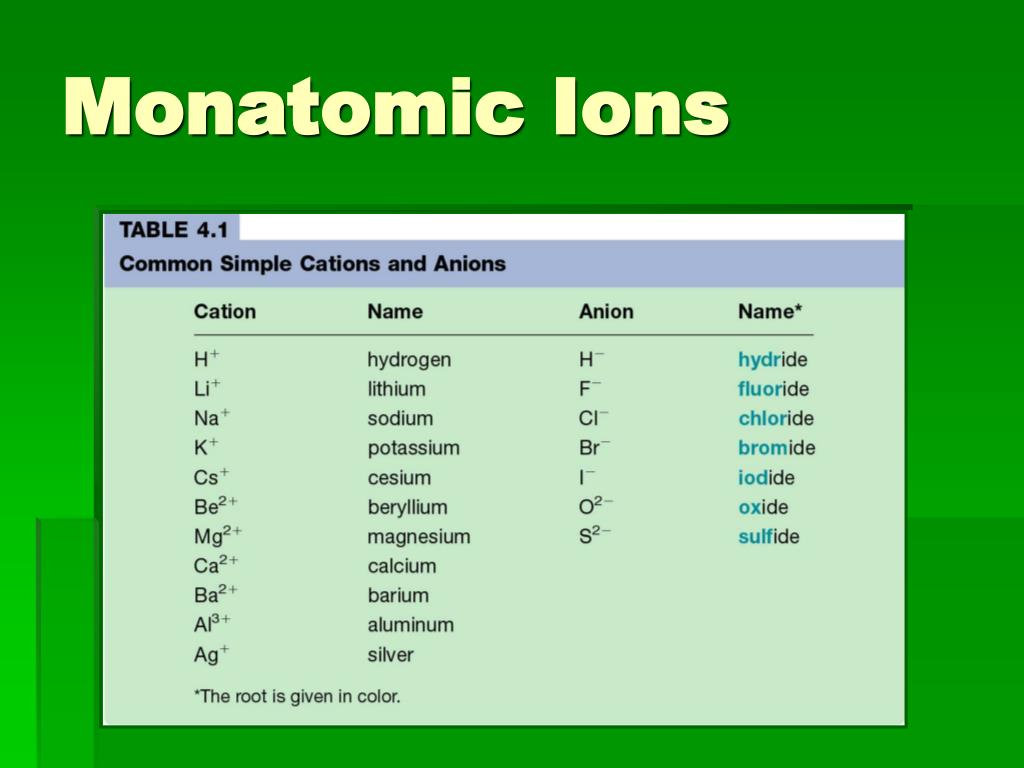
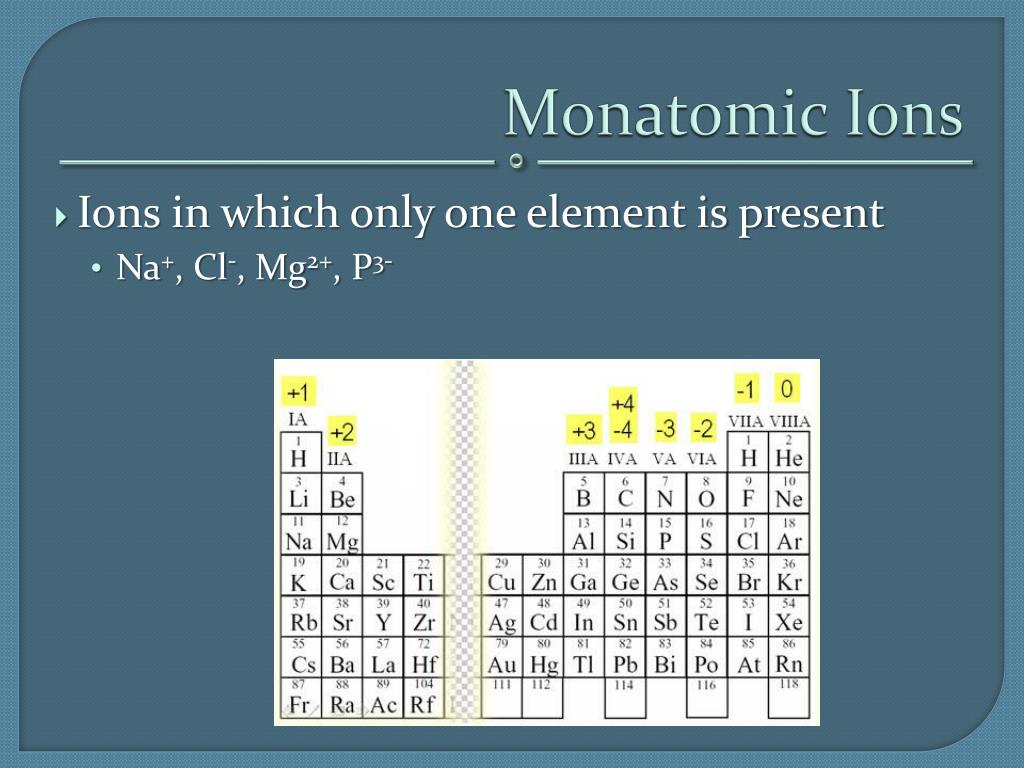
A monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Web about this tutor ›. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. The ion formed by lithium element is explanation: It has one electron more than its atom, so te electron occupies.
PPT Chapter 2 Molecules, Ions, and Compounds PowerPoint Presentation
Web as the most electronegative element, fluorine also cannot be a central atom. Mono atomic ion is defined as the ion which is. Web the most stable monatomic ion will occur when the atom's electron. Web about this tutor ›. Valence shell electron is defined as the.
Monatomic Ion Easy Science Easy science, Ap chemistry, Chemistry
Most frequently, covalent bonds involving. A monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Fluorine will gain one electron. The ion formed by lithium element is explanation: Mono atomic ion is defined as the ion which is.
Web the most stable monatomic ion will occur when the atom's electron. Fluorine will gain one electron. Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen). Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web this property makes organofluorine compounds some of the most stable and inert substances known to man. The stable mono atomic ion of fluorine is. Neutral atom of fluorine has 7 valence electrons. It has one electron more than its atom, so te electron occupies the last p orbital, fills the p shell. Valence shell electron is defined as the. Web forming an octet (eight electrons in the outer shell) provides stability to the atom. Web because fluorine has 7 valence electrons, adding one additional electron will make it the most stable. Web many monatomic ions are found in seawater, including the ions formed from the following list of elements. Web fluorine is a lewis acid in weak acid, which means that it accepts. Though believed not to occur naturally,. Web fluorine is a group 7a element. Web what is the most stable monatomic ion formed from fluorine? Web as the most electronegative element, fluorine also cannot be a central atom. Web cations and anions when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus. Ions are formed when an atom looses or. Web fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent.
Valence Shell Electron Is Defined As The.
Though believed not to occur naturally,. It has one electron more than its atom, so te electron occupies the last p orbital, fills the p shell. Web about this tutor ›. Web as the most electronegative element, fluorine also cannot be a central atom.
Web Many Monatomic Ions Are Found In Seawater, Including The Ions Formed From The Following List Of Elements.
Web the most stable monatomic ion will occur when the atom's electron. A monatomic ion (also called simple ion [1] [2]) is an ion consisting of exactly one atom. Web fluorine (f 2), composed of two fluorine atoms, combines with all other elements except helium and neon to form ionic or covalent. Web fluorine is a lewis acid in weak acid, which means that it accepts.
Web Fluorine Is A Group 7A Element.
Distribute the remaining electrons as lone pairs on the terminal atoms (except hydrogen). Fluorine will gain one electron. The ion formed by lithium element is explanation: Web this property makes organofluorine compounds some of the most stable and inert substances known to man.
Mono Atomic Ion Is Defined As The Ion Which Is.
Web forming an octet (eight electrons in the outer shell) provides stability to the atom. The stable mono atomic ion of fluorine is. Web with other atoms, fluorine forms either polar covalent bonds or ionic bonds. Web cations and anions when a neutral atom loses one or more electrons, the total number of electrons decreases while the number of protons in the nucleus.