What Ions Would Be Formed By X And Y - Web a compound is formed from elements x and y. Which ions will be present in the compound formed when. Magnesium and nitrogen react to form an ionic compound. Each atom tries to m. Web fortunately, the anion is always of known charge and so you can use that to algebraically reverse engineer the principle of charge neutrality. An ion is an atom or group of atoms with a positive or negative charge. Web x° :y° ⁰ (x has one electron, y has 2 on the left, 1 on the right, and 1 on the top and bottom. Which properties are typical of most nonmetals in period three, from na to ar. What are the group numbers of x and y? 5 total) what ions would be.
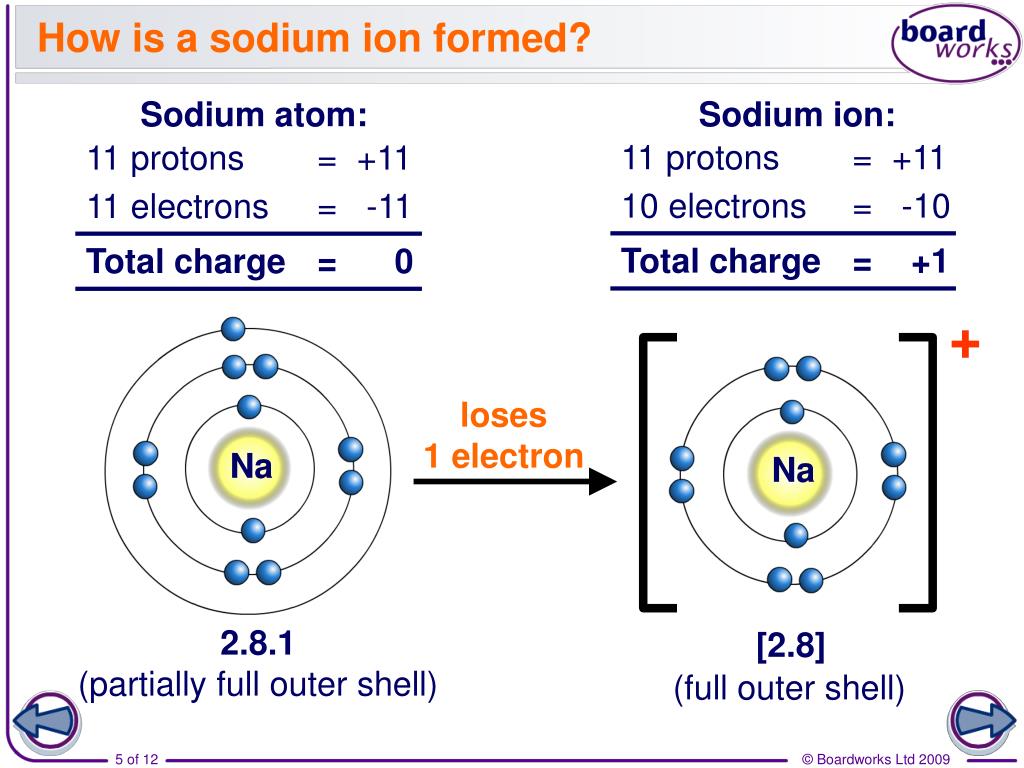
Formation of Ion SPM Chemistry
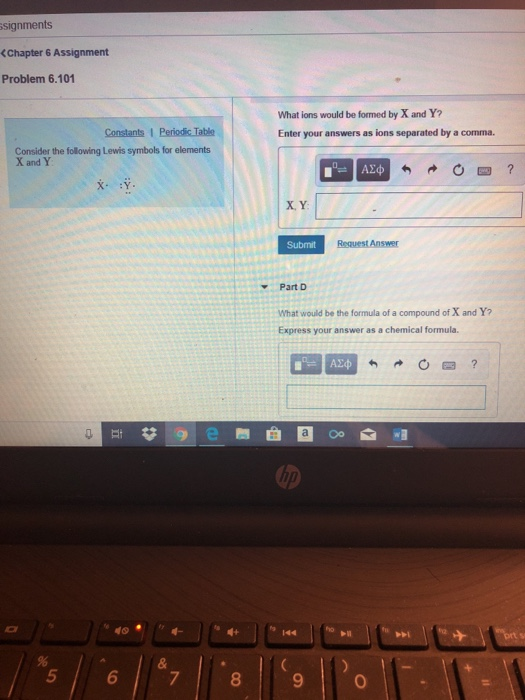
Each atom tries to m. Web 6.101 consider the following lewis symbols for elements x and y: Ions form when atoms lose or gain. Web fortunately, the anion is always of known charge and so you can use that to algebraically reverse engineer the principle of charge neutrality. Which ions will be present in the compound formed when.
Solved Consider the following Lewis symbols for elements X
Web a compound is formed from elements x and y. Web chemistry chemistry questions and answers element x has two valence electrons and element y has 5 valence electrons. Web elements x and y form an ionic compound. 5 total) what ions would be. Web element x is in group 2, and element y in group 7, of the periodic.
TYPE OF IONS YouTube
Web element x is in group 2, and element y in group 7, of the periodic table. Web a compound is formed by elements 'x' (cations) and 'y' (anions). 5 total) what ions would be. It has a giant lattice. An ion is an atom or group of atoms with a positive or negative charge.
Ions
The atoms of a polyatomic ion are tightly bonded together and so the entire ion. Magnesium and nitrogen react to form an ionic compound. 5 total) what ions would be. Ions of 'x' occupy all the tetrahedral voids while ions of 'y'. Ions form when atoms lose or gain.
PPT How do atoms form ions? PowerPoint Presentation ID7021047
Web elements x and y form an ionic compound. Web chemistry chemistry questions and answers element x has two valence electrons and element y has 5 valence electrons. Web a compound is formed from elements x and y. It has a giant lattice. Web a compound is formed by elements 'x' (cations) and 'y' (anions).
Dot Diagram Ionic Bonds Images Amashusho
It has a giant lattice. They form ions by gaining. (atomic number of x is 11 and y is 9) a. Web what ions would be formed by x and y? The atoms of a polyatomic ion are tightly bonded together and so the entire ion.
Solved signments (Chapter 6 Assignment Problem 6.101 What
Web the following diagram shows a set of experimental data points, x, determined when one experimental measurement was. Web 6.101 consider the following lewis symbols for elements x and y: It has a giant lattice. Web x° :y° ⁰ (x has one electron, y has 2 on the left, 1 on the right, and 1 on the top and bottom..
Chem Ions Scientific Tutor
Ions form when atoms lose or gain. Each atom tries to m. An ion is an atom or group of atoms with a positive or negative charge. The atom of x (cations) occupy half of the. (atomic number of x is 11 and y is 9) a.
PPT 1 Name the ions formed by these elements and classify them as
Web what ions would be formed by x and y? Web elements x and y form an ionic compound. It has a giant lattice. Name the compound formed by x and y. Magnesium and nitrogen react to form an ionic compound.
Ions of Elements
Web fortunately, the anion is always of known charge and so you can use that to algebraically reverse engineer the principle of charge neutrality. What are the group numbers of x and y? The atom of x (cations) occupy half of the. Web a compound is formed from elements x and y. (atomic number of x is 11 and y.
Web a compound is formed by elements 'x' (cations) and 'y' (anions). Each atom tries to m. Web elements x and y form an ionic compound. (atomic number of x is 11 and y is 9) a. Q1 as per the levis structure of x and y, x and y has 2 and 7 electrons in their valene shell. 5 total) what ions would be. Ions of 'x' occupy all the tetrahedral voids while ions of 'y'. The atoms of y (anions) form ccp lattice. Which properties are typical of most nonmetals in period three, from na to ar. Which ions will be present in the compound formed when. Web 6.101 consider the following lewis symbols for elements x and y: Web answer (1 of 3): Web chemistry chemistry questions and answers element x has two valence electrons and element y has 5 valence electrons. An ion is an atom or group of atoms with a positive or negative charge. The compound would be xy2 that is one attom of element x combined to form a stable molecule with 2 atoms of. Magnesium and nitrogen react to form an ionic compound. Ions form when atoms lose or gain. The atom of x (cations) occupy half of the. The atoms of a polyatomic ion are tightly bonded together and so the entire ion. They form ions by gaining.
5 Total) What Ions Would Be.
Which ions will be present in the compound formed when. An ion is an atom or group of atoms with a positive or negative charge. Q1 as per the levis structure of x and y, x and y has 2 and 7 electrons in their valene shell. Web element x is in group 2, and element y in group 7, of the periodic table.
The Atom Of X (Cations) Occupy Half Of The.
Web fortunately, the anion is always of known charge and so you can use that to algebraically reverse engineer the principle of charge neutrality. Web elements x and y form an ionic compound. Web a compound is formed from elements x and y. The atoms of a polyatomic ion are tightly bonded together and so the entire ion.
Each Atom Tries To M.
Web a compound is formed by elements 'x' (cations) and 'y' (anions). It has a giant lattice. The compound would be xy2 that is one attom of element x combined to form a stable molecule with 2 atoms of. Web chemistry chemistry questions and answers element x has two valence electrons and element y has 5 valence electrons.
Web What Ions Would Be Formed By X And Y?
(atomic number of x is 11 and y is 9) a. Ions of 'x' occupy all the tetrahedral voids while ions of 'y'. What would be the formula of a compound made up of x and y? Web x° :y° ⁰ (x has one electron, y has 2 on the left, 1 on the right, and 1 on the top and bottom.