Specific Heat Capacity Worksheet Answers - A calorimeter has a heat capacity of 1265 j/oc. Web suitable for gcse and a level physics. It includes a series of questions. Web q = m × c s × δt. Web learn for free regarding math, dexterity, computer design, economics, physics, chemistry, nature, healthcare, accounting,. Where, q is the amount of heat in j, m is the mass, c s is the specific heat capacity of the material in j/g o c , and δt is the change in temperature (t. Web 2 worksheets consisting of 25 questions and answers for the introduction to heat. Web the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Web 147k subscribers subscribe 11 744 views 2 years ago gcse physics worksheet answers explained this video. Web specific heat and heat capacity worksheet directions:
Specific Heat And Heat Capacity Worksheet Answers worksheet
It includes a series of questions. Web specific heat and heat capacity worksheet directions: Web answers = mc∆t, where q = heat energy, m = mass, and ∆t = change in temp. Web pdf, 115.38 kb pdf, 1.48 mb this worksheet is designed for gcse physics students. Web the specific heat capacity of a substance is the energy required to.
30 Specific Heat Worksheet Answer Key Education Template
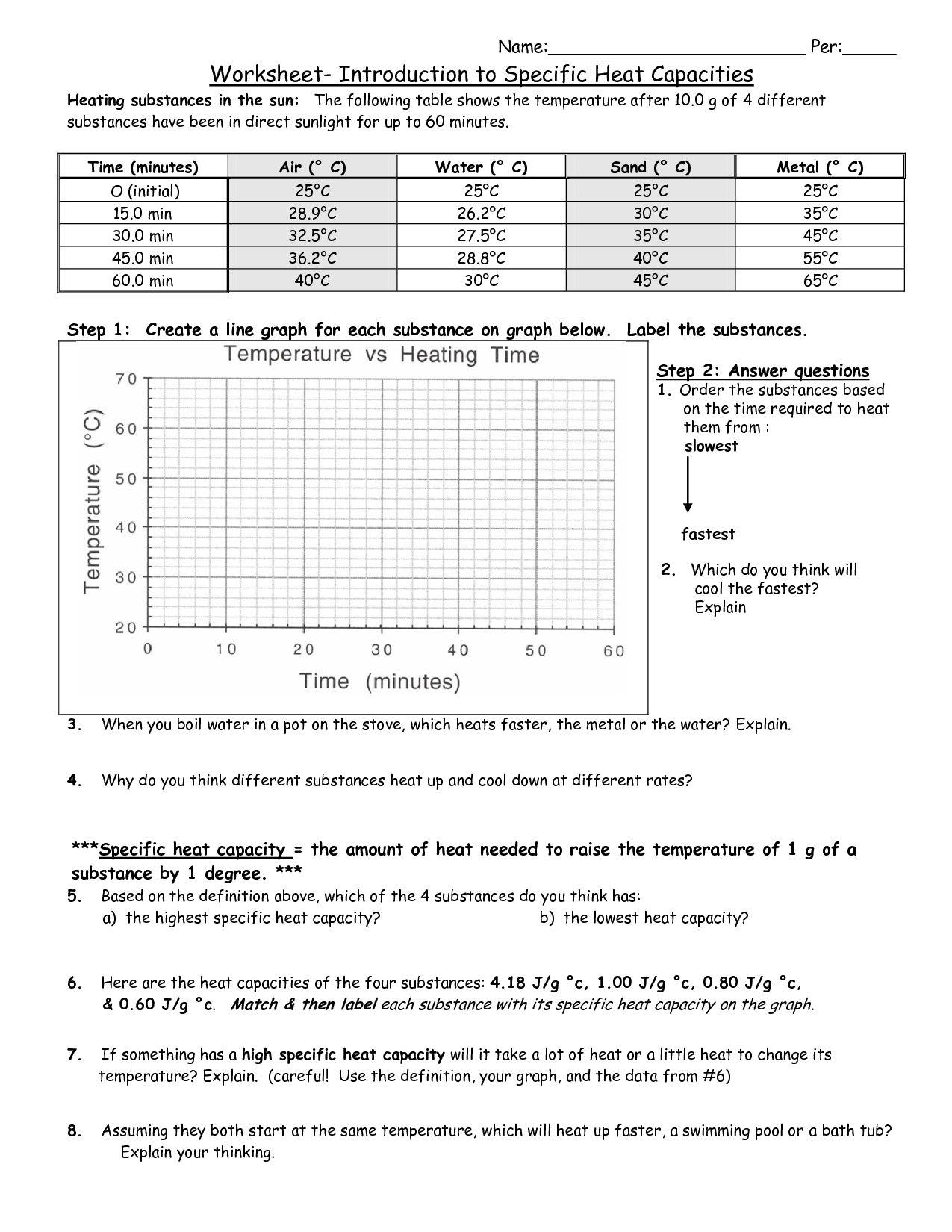
Web 147k subscribers subscribe 11 744 views 2 years ago gcse physics worksheet answers explained this video. 50 assorted questions answers included. Heating substances in the sun: Web the specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one. Web learn for free regarding math, dexterity, computer design,.
30 Specific Heat Worksheet Answer Key Education Template
Web this video explains the answers to the first specific heat capacity gcse physics worksheet. Where, q is the amount of heat in j, m is the mass, c s is the specific heat capacity of the material in j/g o c , and δt is the change in temperature (t. Heating substances in the sun: Because it has a.
14 Best Images of And Specific Heat Capacity Worksheet Specific Heat
This worksheet is for specific heat capacity, shc, calculations at gcse. The following table shows the temperature. Web specific heat capacity subject: Web the specific heat capacity is the amount of heat it takes to change the temperature of one gram of substance by 1°c. Web q = m × c s × δt.
Specific Heat Worksheet Answers
Use q = (m)(cp))(δt) to solve the following problems. Web specific heat and heat capacity worksheet answers with work. This covers specific heat capacity for p1 aqa. Web pdf, 1.51 mb. Web suitable for gcse and a level physics.
30 Specific Heat Worksheet Answer Key Education Template
Lots of practice for using the shc equation. The following table shows the temperature. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Web suitable for gcse and a level physics. Where, q is the amount of heat in j, m is the mass, c s is the specific.
Specific Heat Worksheet Answers
Web 2 worksheets consisting of 25 questions and answers for the introduction to heat. Show all work and units. 50 assorted questions answers included. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. This worksheet is for specific heat capacity, shc, calculations at gcse.
Specific Heat Answers 2013 Heat Capacity
Because it has a higher specific heat capacity 4. Web specific heat and heat capacity worksheet directions: Web 2 worksheets consisting of 25 questions and answers for the introduction to heat. 2 kg of oil and 2 kg of. This covers specific heat capacity for p1 aqa.
30 Specific Heat Worksheet Answer Key Education Template
50 assorted questions answers included. Web learn for free regarding math, dexterity, computer design, economics, physics, chemistry, nature, healthcare, accounting,. Web specific heat and heat capacity worksheet answers with work. 2 kg of oil and 2 kg of. Web pdf, 115.38 kb pdf, 1.48 mb this worksheet is designed for gcse physics students.
14 Best Images of Specific Heat Capacity Worksheet Specific Heat
Use q = (m) (cp) (at) to solve the following problems. Web this video explains the answers to the first specific heat capacity gcse physics worksheet. Use q = (m)(cp))(δt) to solve the following problems. Web pdf, 759.16 kb. It includes a series of questions.
Show all work and units. 2 kg of oil and 2 kg of. Because it has a higher specific heat capacity 4. Web pdf, 1.51 mb. It includes a series of questions. Web q = m × c s × δt. Use q = (m) (cp) (at) to solve the following problems. Heating substances in the sun: Web learn for free regarding math, dexterity, computer design, economics, physics, chemistry, nature, healthcare, accounting,. Web specific heat and heat capacity worksheet directions: Web suitable for gcse and a level physics. The following table shows the temperature. Web specific heat and heat capacity worksheet answers with work. Web 2 worksheets consisting of 25 questions and answers for the introduction to heat. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc. This worksheet is for specific heat capacity, shc, calculations at gcse. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. Where, q is the amount of heat in j, m is the mass, c s is the specific heat capacity of the material in j/g o c , and δt is the change in temperature (t. Web pdf, 759.16 kb. 50 assorted questions answers included.
Because It Has A Higher Specific Heat Capacity 4.
Web this video explains the answers to the first specific heat capacity gcse physics worksheet. Show all work and units. Where, q is the amount of heat in j, m is the mass, c s is the specific heat capacity of the material in j/g o c , and δt is the change in temperature (t. A reaction causes the temperature of the calorimeter to change from 22.34oc to 25.12oc.
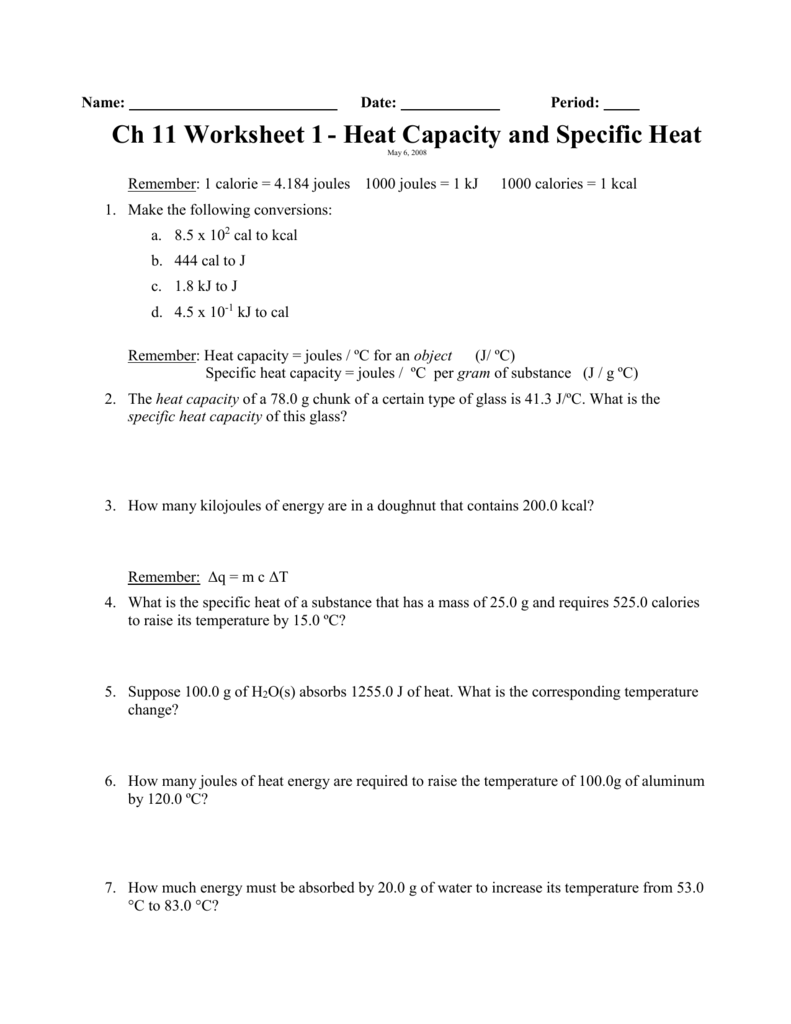
Web Answers = Mc∆T, Where Q = Heat Energy, M = Mass, And ∆T = Change In Temp.
2 kg of oil and 2 kg of. This covers specific heat capacity for p1 aqa. Web 2 worksheets consisting of 25 questions and answers for the introduction to heat. Lots of practice for using the shc equation.
Heating Substances In The Sun:
Web learn for free regarding math, dexterity, computer design, economics, physics, chemistry, nature, healthcare, accounting,. Web specific heat and heat capacity worksheet 1 the temperature of 335 g of water changed from 24.5oc to 26.4oc. It includes a series of questions. This worksheet is for specific heat capacity, shc, calculations at gcse.
Web The Specific Heat Capacity Is The Amount Of Heat It Takes To Change The Temperature Of One Gram Of Substance By 1°C.
The following table shows the temperature. Web the specific heat capacity of a substance is the energy required to raise the temperature of one gram of the substance by one. Web 147k subscribers subscribe 11 744 views 2 years ago gcse physics worksheet answers explained this video. Use q = (m)(cp))(δt) to solve the following problems.