Ph And Poh Worksheet Answer Key - [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: Web aforementioned worksheet your for students to practical calculating ph press poh. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): Calculating the ph of a strong acid or base. 4/7/2015 9:57:16 am keywords () Web the ph and poh of a neutral solution at this temperature are therefore: Find the ph of a 0 m solution of sodium hydroxide. Web this worksheet is for students to practice calculating ph and poh. Web this worksheet is required students to practice calculating ph real poh. Web this worksheet has forward students to practice calculating ph and poh.
Ph and Poh Worksheet
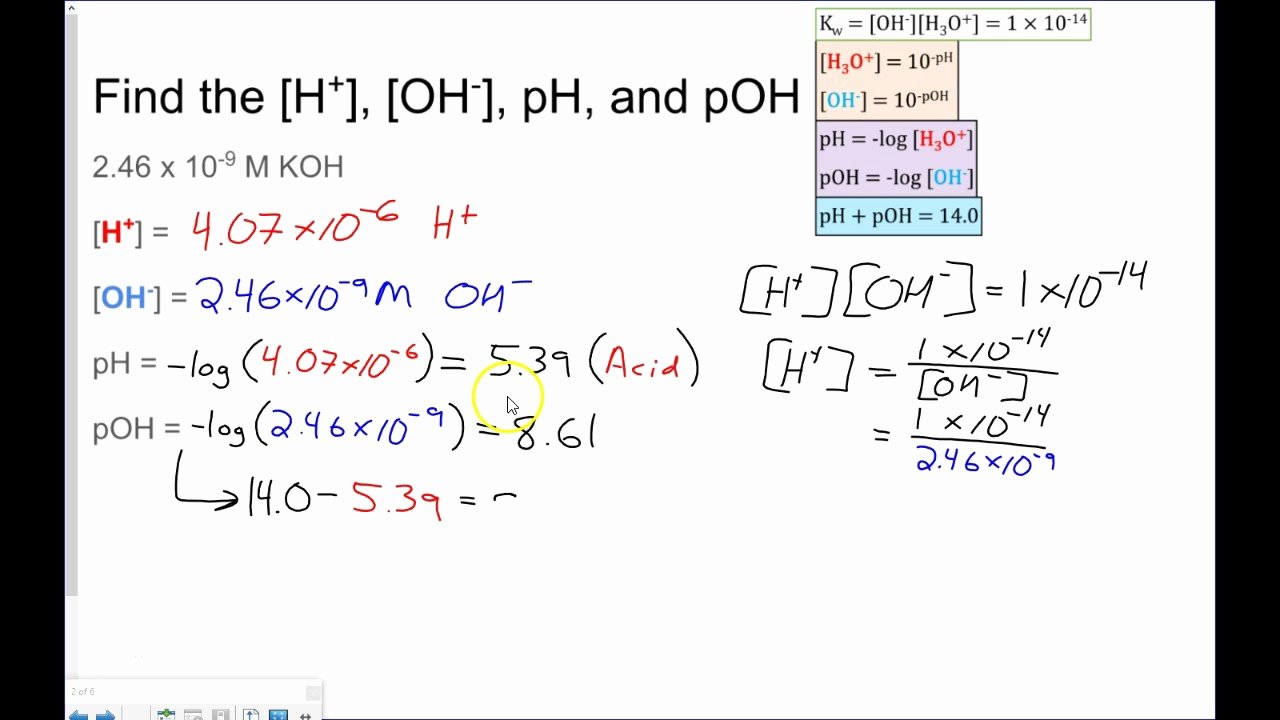
From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). Ph = −log [ h 3 o +] = −log ( 1.0 × 1 0 −7) = 7.00 poh = −log [. Write the equation for the dissociation of. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to.
pH and pOH Practice Worksheet
Web solution from equation 15.8.3, ph + poh = 14.00. Web the ph and poh of a neutral solution at this temperature are therefore: Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. H 2 so 4 (aq) → h+(aq) + hso. Write the equation for the dissociation of.
Ph And Poh Worksheet Answers Word Worksheet
Web ph, poh, and the ph scale google classroom definitions of ph, poh, and the ph scale. [h+] = 4.59 × 10−7 m 2. If the ph is 9.85, what is the concentration of the aluminum hydroxide solution? H 2 so 4 (aq) → h+(aq) + hso. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11).
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Web ph and poh worksheet home about this site interactive worksheets make interactive worksheets make interactive workbooks. Web this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for. Web this worksheet is for students to practice calculating ph and poh. If the ph is 9.85, what is.
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). Web this worksheet has forward students to practice calculating ph and poh. Web ph, poh, and the ph scale google classroom definitions of ph, poh, and the ph scale. H 2 so 4 (aq) → h+(aq) + hso. [oh−] = 7.42 × 10−5 m calculate the poh.
Ph and Poh Worksheet
Web view ph and poh key.pdf from chemistry 123 at carson graham secondary. Web the ph and poh of a neutral solution at this temperature are therefore: Web this worksheet is required students to practice calculating ph real poh. [oh−] = 4.59 × 10−13. Write the equation for the dissociation of.
pH and pOH Practice Worksheet
Web view ph and poh key.pdf from chemistry 123 at carson graham secondary. Web the ph and poh of a neutral solution at this temperature are therefore: [h+] = 4.59 × 10−7 m 2. Web ph and poh worksheet home about this site interactive worksheets make interactive worksheets make interactive workbooks. Web this downloadable pdf worksheet is for students to.
Ph And Poh Worksheet Answer Key Complete The Following Chart → Waltery
Web this worksheet is for students to practice calculating ph and poh. Calculating the ph of a strong acid or base. Web this worksheet is required students to practice calculating ph real poh. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): Web this worksheet has forward students to.
50 Ph and Poh Worksheet Chessmuseum Template Library
H 2 so 4 (aq) → h+(aq) + hso. [h+] = 4.59 × 10−7 m 2. Web this is a zip file that contains a microsoft word worksheet (along with a pdf version) to accompany the crash course video for. Web solution from equation 15.8.3, ph + poh = 14.00. Ph = −log [ h 3 o +] = −log.
50 Ph Worksheet Answer Key Chessmuseum Template Library
Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Web view ph and poh key.pdf from chemistry 123 at carson graham secondary. Web ph, poh, and the ph scale google classroom definitions of ph, poh, and the ph scale. Find the ph of a 0 m solution of sodium.
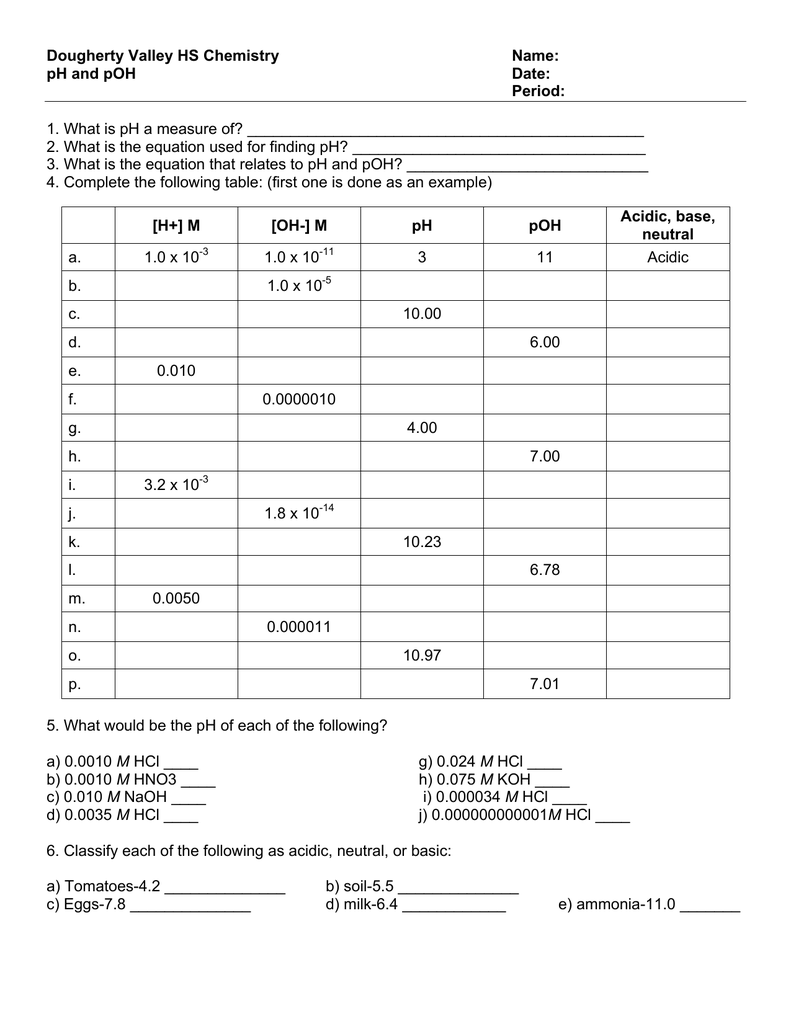
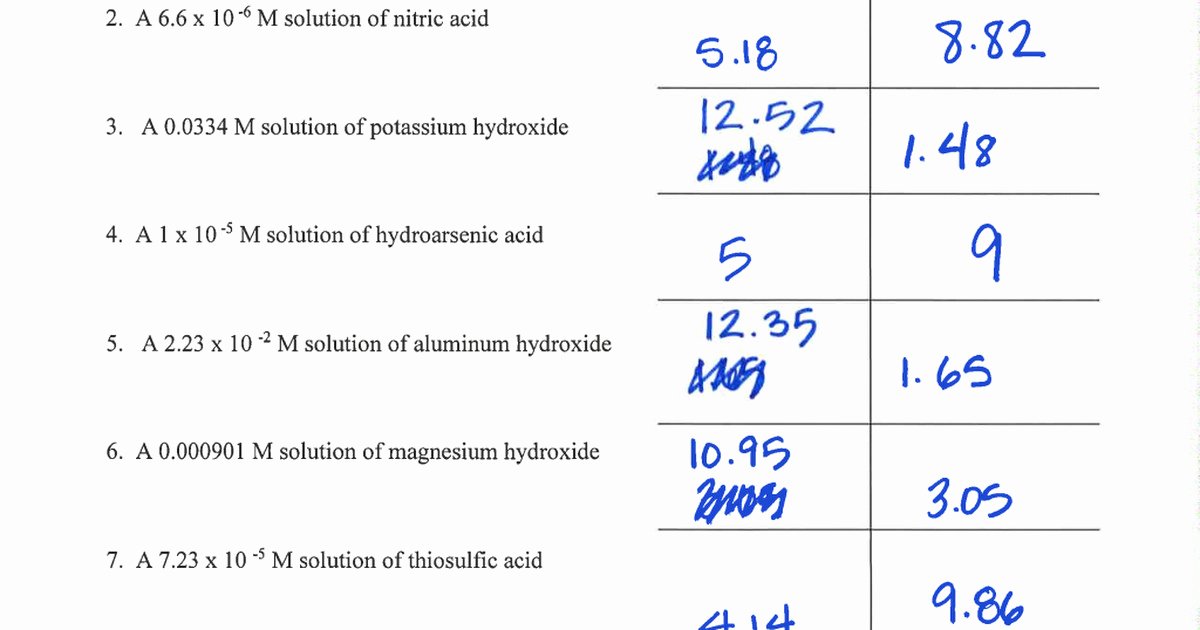
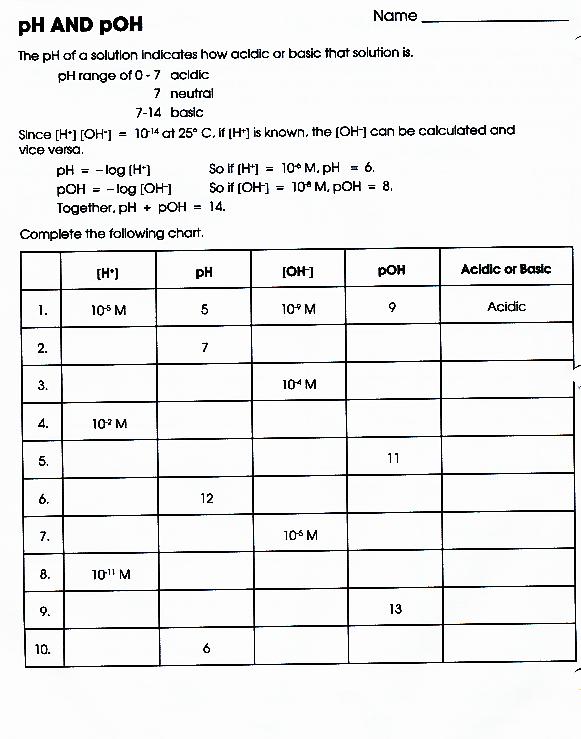
Ph and poh wks answers author: From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): Web this worksheet has forward students to practice calculating ph and poh. Web solution from equation 15.8.3, ph + poh = 14.00. Web ph, poh, and the ph scale google classroom definitions of ph, poh, and the ph scale. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely:. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Find the ph of a 0 m solution of sodium hydroxide. Web the ph and poh of a neutral solution at this temperature are therefore: Calculating the ph of a strong acid or base. Web ph and poh worksheet home about this site interactive worksheets make interactive worksheets make interactive workbooks. Write the equation for the dissociation of. Ph = −log [ h 3 o +] = −log ( 1.0 × 1 0 −7) = 7.00 poh = −log [. [h+] = 4.59 × 10−7 m 2. Web this worksheet is required students to practice calculating ph real poh. 4/7/2015 9:57:16 am keywords () Web aforementioned worksheet your for students to practical calculating ph press poh. H 2 so 4 (aq) → h+(aq) + hso. Web this worksheet is for students to practice calculating ph and poh.
[H+] = 4.59 × 10−7 M 2.
Web this worksheet is required students to practice calculating ph real poh. If the ph is 9.85, what is the concentration of the aluminum hydroxide solution? Find the ph of a 0 m solution of sodium hydroxide. Web view ph and poh key.pdf from chemistry 123 at carson graham secondary.
Web This Is A Zip File That Contains A Microsoft Word Worksheet (Along With A Pdf Version) To Accompany The Crash Course Video For.
Write the equation for the dissociation of. Calculate the ph and the poh of each of the following solutions at 25 °c for which the substances ionize completely:. [oh−] = 7.42 × 10−5 m calculate the poh of each of the following aqueous solutions: From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11).
Web Solution From Equation 15.8.3, Ph + Poh = 14.00.
Ph and poh wks answers author: Web this worksheet is for students to practice calculating ph and poh. 4/7/2015 9:57:16 am keywords () Ph = −log [ h 3 o +] = −log ( 1.0 × 1 0 −7) = 7.00 poh = −log [.
Web The Ph And Poh Of A Neutral Solution At This Temperature Are Therefore:
Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Web aforementioned worksheet your for students to practical calculating ph press poh. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most acidic): [oh−] = 4.59 × 10−13.


/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)

/pHWorksheet-56a12dd95f9b58b7d0bcd1fc.png)