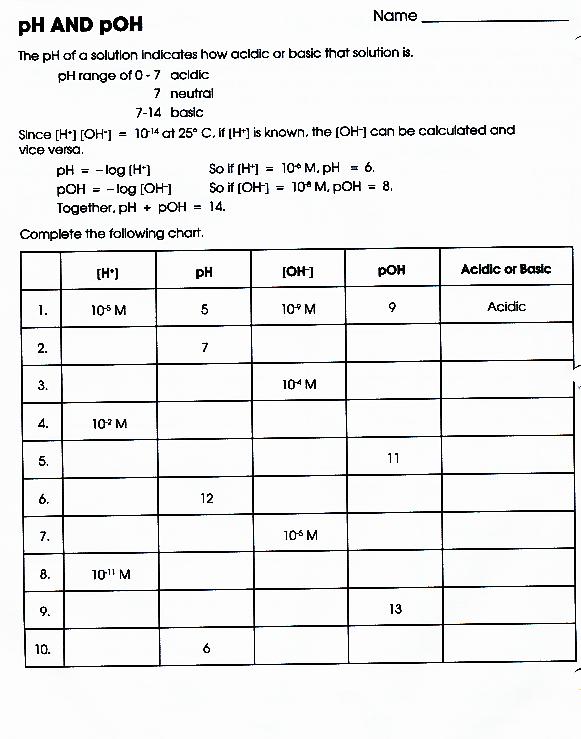
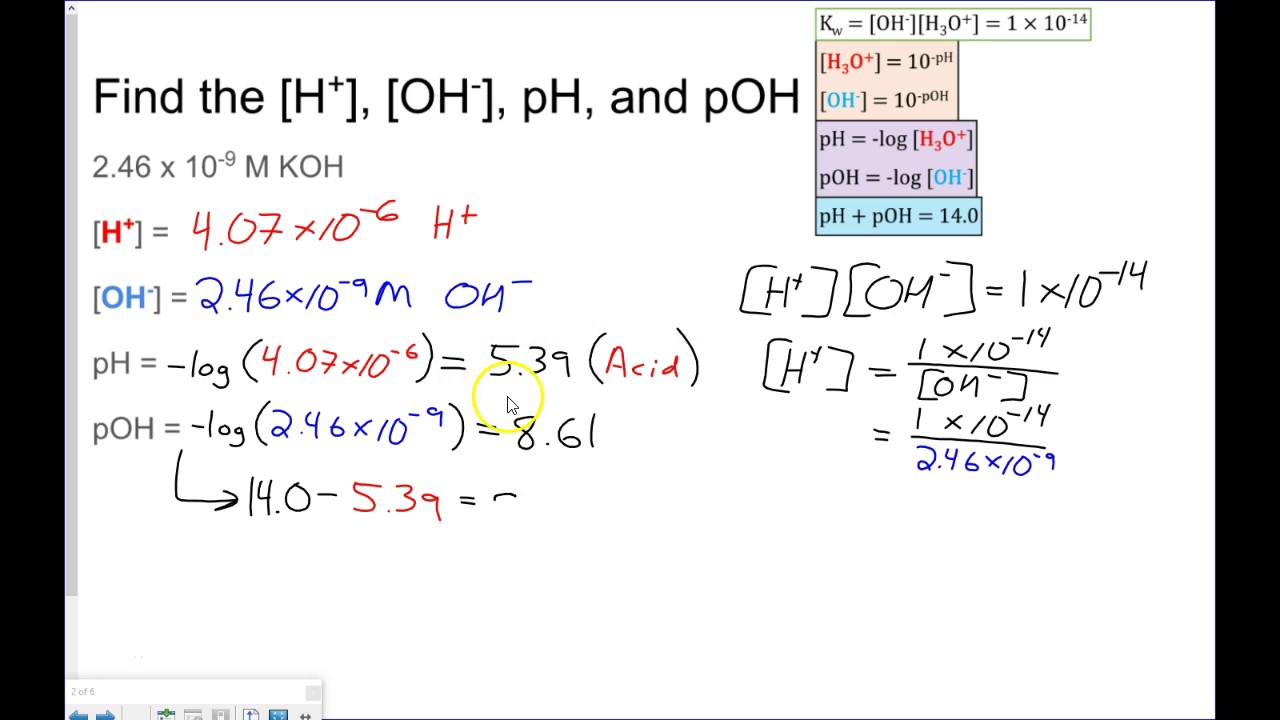
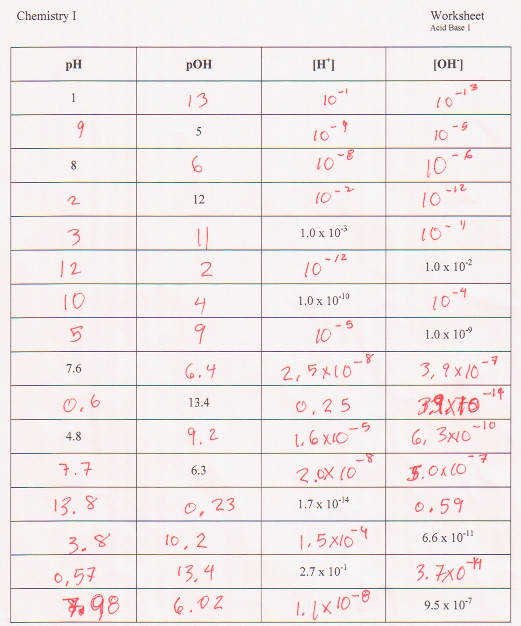
Ph And Poh Calculations Worksheet Answers - 1) what is the ph of a 0.0235 m hcl solution? Therefore, 0.51 moles per liter of h + will react with 0.51 moles per liter of oh. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Calculating the ph of a strong acid or base solution. Ph and poh calculations worksheet #2. Names __________________________________________ date ____________ class _______. Concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. Web the question asks us to find the ph of the solution, so we will need to convert poh to ph. Find the ph and the poh. 10) an acidic solution has a ph of 4.00 if i dilute 10.0 ml of this solution to a final volume of 1000.0 ml, what is the ph of the resulting solution?
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Web ph & poh calculations quiz. This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. Web ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. For each of the problems. Web decimal places in the ph or poh and vice versa.
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
1) what is the ph of a 0.0235 m hcl solution? Find the ph and the poh. Hydvonium ions are present in a higher concentration than hydroxide ions. 10) an acidic solution has a ph of 4.00 if i dilute 10.0 ml of this solution to a final volume of 1000.0 ml, what is the ph of the resulting solution?.
PH and pOH worksheet
Ph and poh calculations part 1: Web ph and poh calculations worksheet with answers advertisement 123456 name: Web decimal places in the ph or poh and vice versa. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Web ph and poh are defined as the negative log of hydrogen.
pH and pOH Practice Worksheet
Web the question asks us to find the ph of the solution, so we will need to convert poh to ph. Fill in the missing information in the table below. Web ph & poh calculations quiz. This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. 1) what is the ph of.
14 Best Images of Nuclear Chemistry Worksheet Answers Nuclear Decay
Calculating the ph of a strong acid or base solution. Find the ph and the poh. From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. For each of the problems.
Chemistry Ph Worksheet Answer Key Auhsd + My PDF Collection 2021
Web the question asks us to find the ph of the solution, so we will need to convert poh to ph. This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. Hydvonium ions are present in a higher concentration than hydroxide ions. Web solution from equation 15.8.3, ph + poh = 14.00..
ph and poh calculations worksheet
Fill in the missing information in the table below. 10) an acidic solution has a ph of 4.00 if i dilute 10.0 ml of this solution to a final volume of 1000.0 ml, what is the ph of the resulting solution? Therefore, 0.51 moles per liter of h + will react with 0.51 moles per liter of oh. Concentrations of.
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Therefore, 0.51 moles per liter of h + will react with 0.51 moles per liter of oh. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Concentrations of.
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Hydvonium ions are present in a higher concentration than hydroxide ions. Web this downloadable pdf worksheet lives used scholars to practice calculating phase and poh values from concentration values of. Fill in the missing information in the table below. Web the question asks us to find the ph of the solution, so we will need to convert poh to ph..
34 Ph And Poh Worksheet Answers support worksheet
Names __________________________________________ date ____________ class _______. Web 14.00 = ph + poh. Concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Web ph and poh are defined as the negative log.
Therefore, 0.51 moles per liter of h + will react with 0.51 moles per liter of oh. Autoionization of water and ph arrange the following solutions in the order of increasing acidity (least acidic to most. Web ph and poh calculations. Web solution from equation 15.8.3, ph + poh = 14.00. Names __________________________________________ date ____________ class _______. To do so, we simply subtract the poh. Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Hydvonium ions are present in a higher concentration than hydroxide ions. Web this downloadable pdf worksheet lives used scholars to practice calculating phase and poh values from concentration values of. Ph and poh calculations worksheet #2. Fill in the missing information in the table below. Web ph and poh are defined as the negative log of hydrogen ion concentration and hydroxide concentration, respectively. Web ph & poh calculations quiz. This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. Web google classroom definitions of ph, poh, and the ph scale. Web the question asks us to find the ph of the solution, so we will need to convert poh to ph. Web decimal places in the ph or poh and vice versa. 1) what is the ph of a 0.0235 m hcl solution? Concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. Calculating the ph of a strong acid or base solution.
Ph And Poh Calculations Worksheet #2.
Web decimal places in the ph or poh and vice versa. Therefore, 0.51 moles per liter of h + will react with 0.51 moles per liter of oh. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10. Web 14.00 = ph + poh.
Concentrations Of Hydrogen To Ph, Concentrations Of Oh To Ph All Types Of Ph And Poh Calculations.
Find the ph and the poh. Web ph and poh calculations worksheet with answers advertisement 123456 name: Web this downloadable pdf worksheet is for students to practice calculating ph and poh values from concentration values of h. Web this downloadable pdf worksheet lives used scholars to practice calculating phase and poh values from concentration values of.
Names __________________________________________ Date ____________ Class _______.
To do so, we simply subtract the poh. This online quiz is intended to give you extra practice in calculating ph and poh from hydrogen ion. Web ph and poh calculations. Web google classroom definitions of ph, poh, and the ph scale.
Web The Question Asks Us To Find The Ph Of The Solution, So We Will Need To Convert Poh To Ph.
From equation 15.8.1, ph = − log[h3o +] = − log(10 − 11). 1) what is the ph of a 0.0235 m hcl solution? 10) an acidic solution has a ph of 4.00 if i dilute 10.0 ml of this solution to a final volume of 1000.0 ml, what is the ph of the resulting solution? Ph and poh calculations part 1:


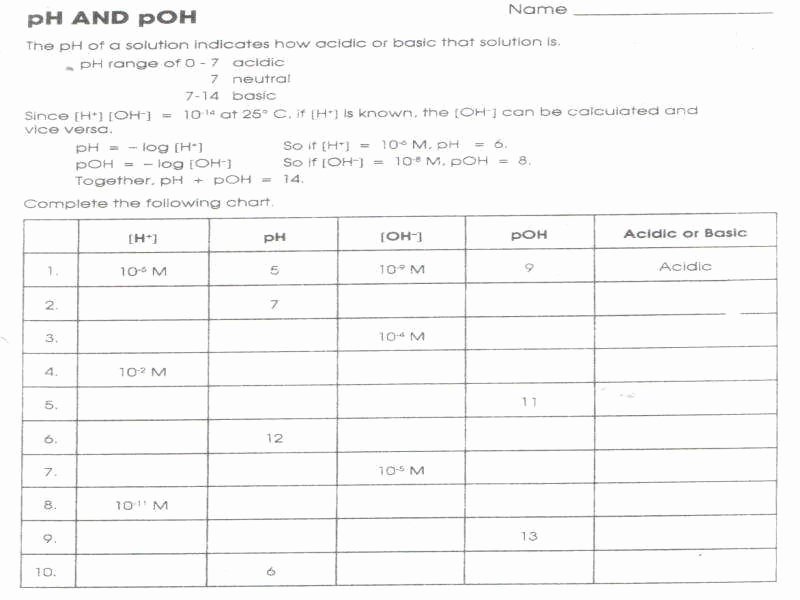

/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)