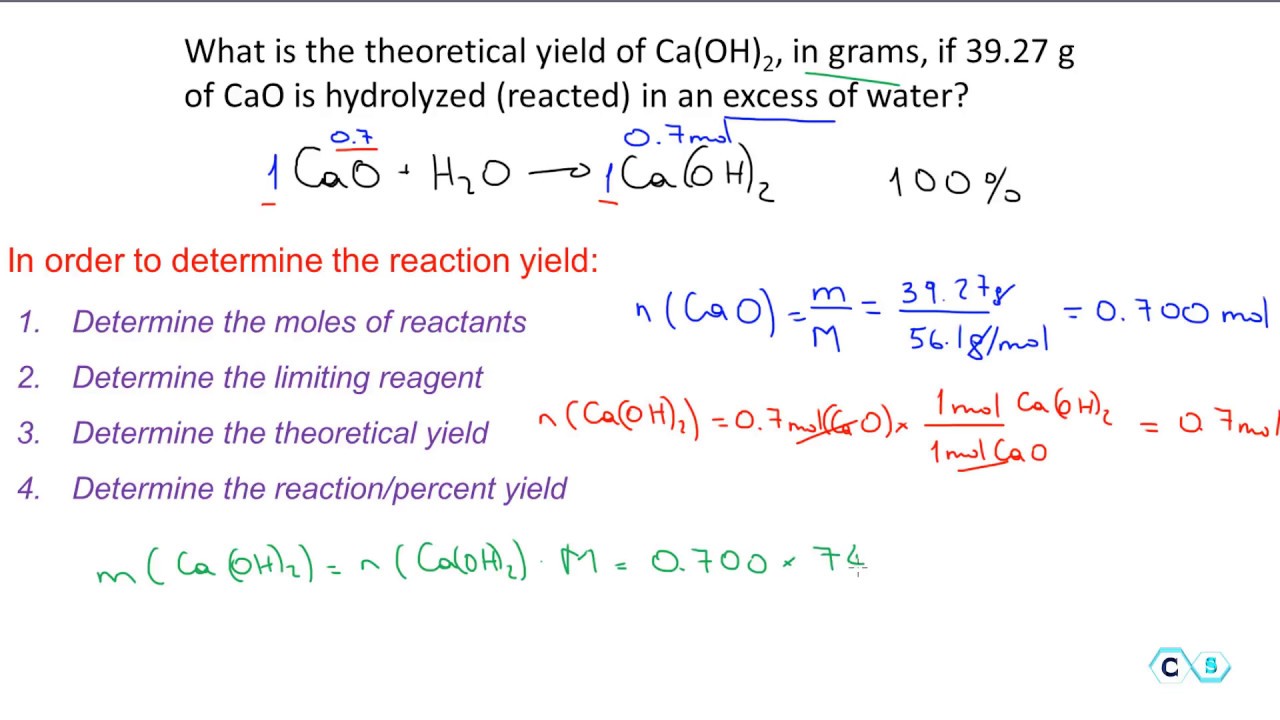
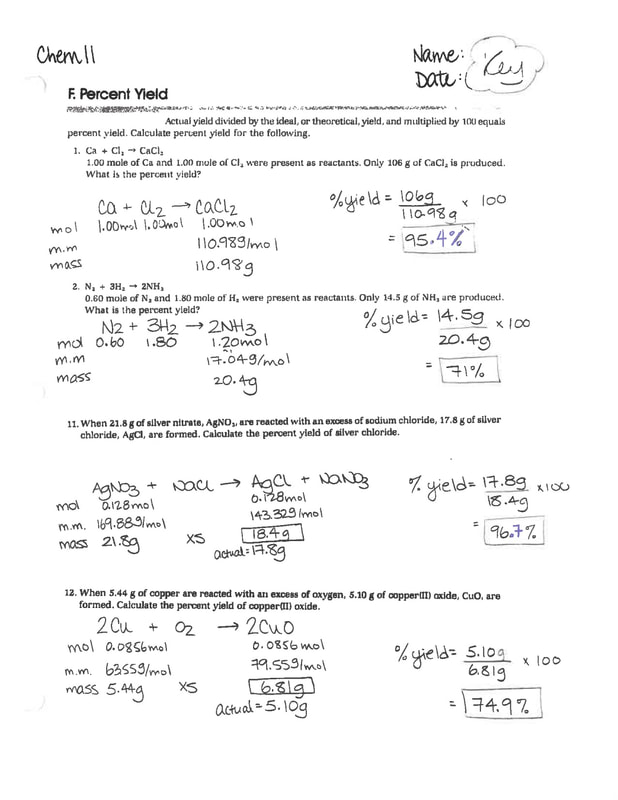
Percent Yield Worksheet Answers - If the actual yield is 63.7 g of chlorobenzene, calculate the percent. Web remember to use the balanced chemical reaction: Choose an answer and hit 'next'. Web calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100. 4.93 × 10 −5 l or 49.3 μl. Web the percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage:. Worksheets are percent yield work, work percent yield name,. Question 1 of 3 if the reaction of 6.5. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass. 5) if i do this reaction with 15 grams.
Limiting Reagents and Percentage Yield Worksheet answers.doc Zinc
Web the percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage:. A revision homework or class worksheet with answers that covers percentage yield in c3 gcse. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass. Web learn about the percent yield of chemical reactions..
Percent Actual And Theoretical Yield Worksheet Answers With Work Tuts
Choose an answer and hit 'next'. If the actual yield is 63.7 g of chlorobenzene, calculate the percent. Web theoretical yeild theoretical yield = answer to your stoich problem. Any yield under 100% is reasonable under the law of conservation of mass. Web the percent yield is the ratio of the actual yield to the theoretical yield, expressed as a.
Ms McLarty's Classes Chem 11
Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a. Any yield under 100% is reasonable under the law of conservation of mass. A revision homework or class worksheet with answers that covers percentage yield in c3 gcse. 2mg (s) + o2 (g) → 2mgo (s). Na 2.
Quiz & Worksheet How to Calculate Percent Yield
Worksheets are percent yield work, work percent yield name,. 2mg (s) + o2 (g) → 2mgo (s). Question 1 of 3 if the reaction of 6.5. What is the theoretical yield if 45.6 g of benzene react? Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and.
Theoretical and Percent Yield Worksheet Answers
What is the theoretical yield if 45.6 g of benzene react? Choose an answer and hit 'next'. Worksheets are percent yield work, work percent yield name,. Web remember to use the balanced chemical reaction: Web theoretical yeild theoretical yield = answer to your stoich problem.
30++ Percent Yield Worksheet Answers
A revision homework or class worksheet with answers that covers percentage yield in c3 gcse. Web theoretical yeild theoretical yield = answer to your stoich problem. Web mol fe 533 g fe % yield actual yield 100 theoretica l yield % yield 464 g fe 100 87% 533 g fe 3. 4.93 × 10 −5 l or 49.3 μl. Complete.
Limiting Reactant Worksheet Answers
Check the answers and the solutions below. Web learn about the percent yield of chemical reactions. 5) if i do this reaction with 15 grams. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass. 1 % yield = actual yield x 100 theoretical yield balance.
Theoretical and Percent Yield Worksheet Answers
If the actual yield is 63.7 g of chlorobenzene, calculate the percent. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the limiting reactant and determining the amounts of reactants needed at a given percent yield. Web po 4 (nh 4 3 po 4 + nano 3 which reactant is limiting, assuming.
Stoichiometry Percent Yield Calculations Worksheet Answers CUALACT
1 % yield = actual yield x 100 theoretical yield balance. A revision homework or class worksheet with answers that covers percentage yield in c3 gcse. Worksheets are percent yield work, work percent yield name,. Web calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100. 2mg (s) + o2 (g) → 2mgo.
Limiting Reactant And Percent Yield Worksheet Answer Key —
Na 2 c 2 o 4 is the. What is the theoretical yield if 45.6 g of benzene react? Web theoretical yeild theoretical yield = answer to your stoich problem. Web learn about the percent yield of chemical reactions. Any yield under 100% is reasonable under the law of conservation of mass.
Web po 4 (nh 4 3 po 4 + nano 3 which reactant is limiting, assuming we started with 30.0 grams of ammonium nitrate and 50.0. Web mol fe 533 g fe % yield actual yield 100 theoretica l yield % yield 464 g fe 100 87% 533 g fe 3. In examples \(\pageindex{1}\) and \. Complete all sections of this report and answer all questions in complete sentences for full. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the limiting reactant and determining the amounts of reactants needed at a given percent yield. You will receive your score and answers at the end. Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass. Web solutions 1) write a balanced equation for the reaction of tin (iv) phosphate with sodium carbonate to make tin (iv) carbonate and. Check the answers and the solutions below. A revision homework or class worksheet with answers that covers percentage yield in c3 gcse. Any yield under 100% is reasonable under the law of conservation of mass. Web pdf, 190.36 kb. The percent yield of a reaction is the ratio. 4.93 × 10 −5 l or 49.3 μl. Web a selection of worksheets together with answer sheets which are aimed at chemistry students for use in class. Actual yield = given in the problem or the experimental yield. Question 1 of 3 if the reaction of 6.5. 1 % yield = actual yield x 100 theoretical yield balance. Web calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100. If the actual yield is 63.7 g of chlorobenzene, calculate the percent.
Web Solutions 1) Write A Balanced Equation For The Reaction Of Tin (Iv) Phosphate With Sodium Carbonate To Make Tin (Iv) Carbonate And.
Web chemistry 1 volume 2 worksheet 12 percent yield in chemical reactions calculate the percent yield of a reaction that had a. 2mg (s) + o2 (g) → 2mgo (s). You will receive your score and answers at the end. 4.93 × 10 −5 l or 49.3 μl.
The Percent Yield Of A Reaction Is The Ratio.
If the actual yield is 63.7 g of chlorobenzene, calculate the percent. In examples \(\pageindex{1}\) and \. Worksheets are percent yield work, work percent yield name,. Web the percent yield is the ratio of the actual yield to the theoretical yield, expressed as a percentage:.
5) If I Do This Reaction With 15 Grams.
Na 2 c 2 o 4 is the. Web learn about the percent yield of chemical reactions. Actual yield = given in the problem or the experimental yield. The practice problems will address finding the percent yield from a single reactant, from two reactants considering the limiting reactant and determining the amounts of reactants needed at a given percent yield.
A Revision Homework Or Class Worksheet With Answers That Covers Percentage Yield In C3 Gcse.
Web calculate the percent yield by dividing the actual yield by the theoretical yield and multiplying by 100. What is the theoretical yield if 45.6 g of benzene react? Complete all sections of this report and answer all questions in complete sentences for full. Choose an answer and hit 'next'.