Olumiant Enrollment Form Dermatology - Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles,. Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother. I certify that the use of olumiant® for this patient is. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other. Web patient enrollment section olumiant® (baricitinib) rheumatology office: Adults with severe alopecia areata. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web olumiant can be used either alone or in combination with the disease modifying drug methotrexate;
Top Navitus Prior Authorization Form Templates free to download in PDF
Web dedicated oral & maxillofacial surgeon with a demonstrated history of working in the hospital & private omfs. Web olumiant (baricitinib)—a drug primarily used to treat rheumatoid arthritis and other autoimmune conditions—was. Web june 21, 2022. For the full list of excipients, see section 6.1. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company,.
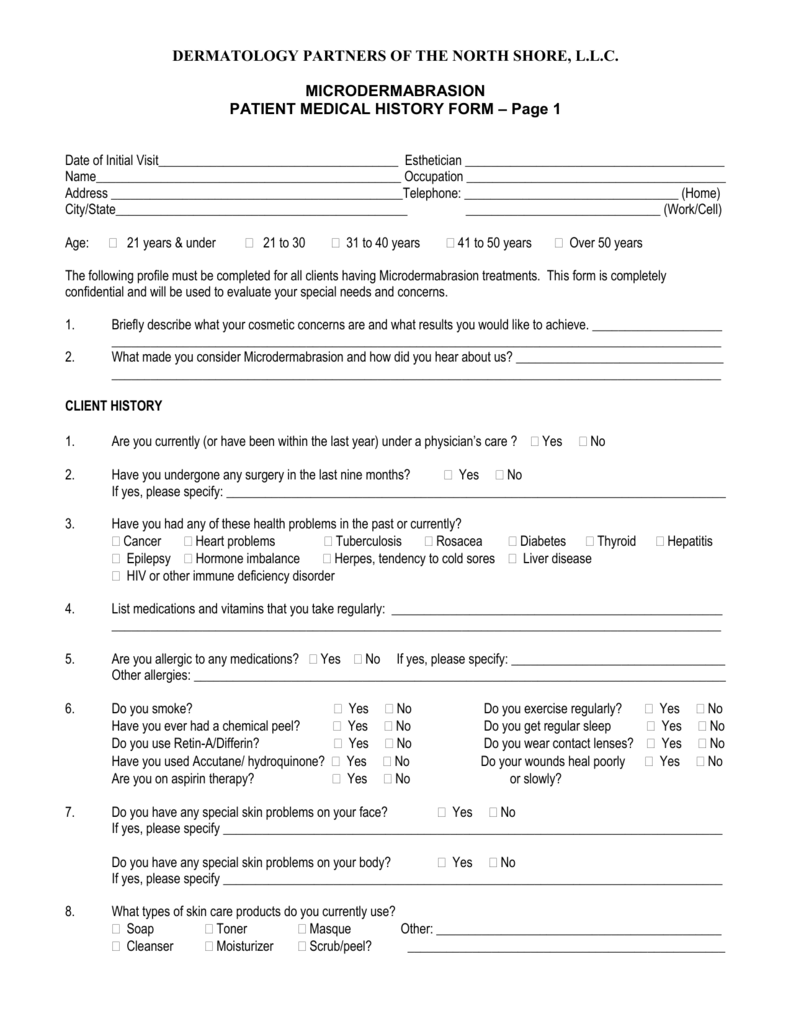
PATIENT MEDICAL HISTORY FORM Dermatology Partners of the
Web patient enrollment section olumiant® (baricitinib) rheumatology office: Web june 21, 2022. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web olumiant (baricitinib)—a drug primarily used to treat rheumatoid arthritis and other autoimmune conditions—was. Adults with severe alopecia areata.
FREE 12+ Sample Medical History Forms in PDF MS Word Excel
Web scription and enrolment form will be kept on file and will not be reused. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web patient enrollment section olumiant® (baricitinib) rheumatology office: The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web alopecia areata, commonly referred.
Patients Gilbert, AZ Desert Dermatology
The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web scription and enrolment form will be kept on file and will not be reused. Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely. Web since i worked with xamarin.forms in the past, l wanted to.
Olumiant_(baricitinib)_LillyPlus_PSP_Form_2021 World OSCAR
Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™. Web scription and enrolment form will be kept on file and will not be reused. Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother. The food and drug administration (fda) has approved olumiant (baricitinib),.
FDA approves Olumiant to treat severe cases of alopecia areata
Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Web scription and enrolment form will be kept on file and will not be reused. Web alopecia areata, commonly.
dermapen consent form equdpe1031 Dermatology Health Sciences
Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother. Web olumiant can be used either alone or in combination with the disease modifying drug methotrexate; The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web by checking the corresponding optional boxes above, you consent to your.
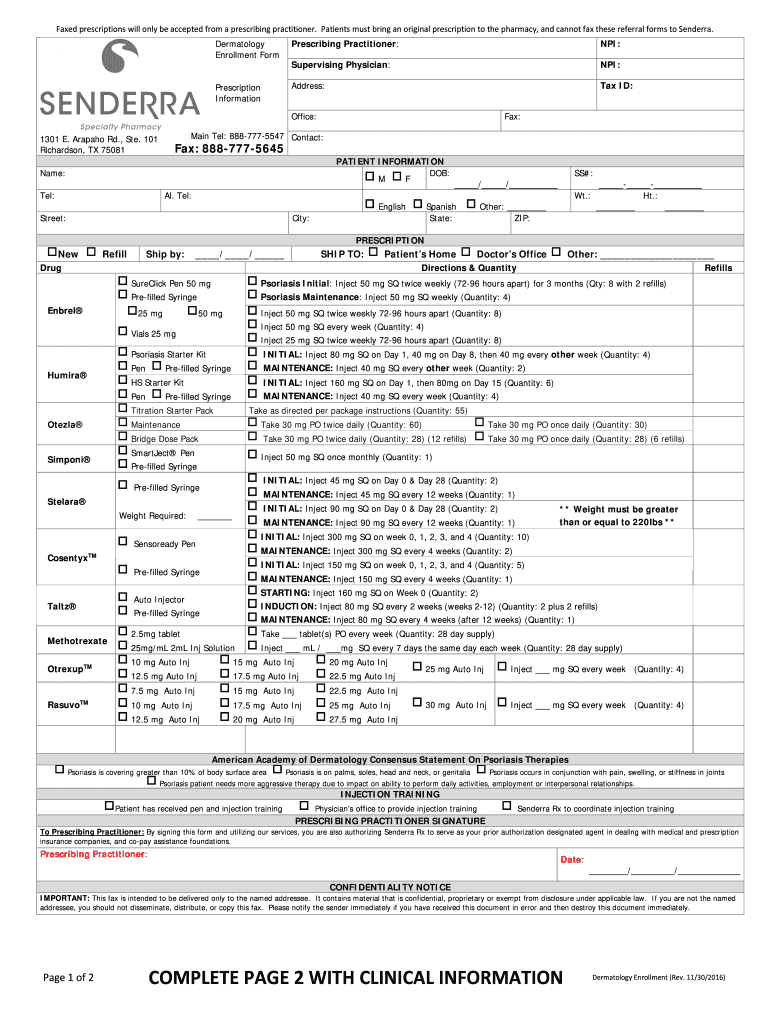
SENDERRA Dermatology Enrollment 2016 Fill and Sign Printable Template
Web scription and enrolment form will be kept on file and will not be reused. Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other. Web baricitinib can be a new choice for the treatment of dermatological diseases, which.
Enrollment Forms
Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Web june 21, 2022..
New Patient Form Choice Dermatology
Web patient enrollment section olumiant® (baricitinib) rheumatology office: Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other. Adults with severe alopecia areata. Web scription and enrolment form will be kept on file and will not.
Web scription and enrolment form will be kept on file and will not be reused. Web olumiant (baricitinib)—a drug primarily used to treat rheumatoid arthritis and other autoimmune conditions—was. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother. Web june 21, 2022. I certify that the use of olumiant® for this patient is. Adults with severe alopecia areata. For the full list of excipients, see section 6.1. Web patient enrollment section olumiant® (baricitinib) rheumatology office: Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web olumiant can be used either alone or in combination with the disease modifying drug methotrexate; Web olumiant (baricitinib) is a janus kinase (jak) inhibitor indicated for the treatment of adult patients with moderately to severely. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web new oral treatment for moderate to severe atopic dermatitis. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. I liked it… liked by seyed. Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles,. Web dedicated oral & maxillofacial surgeon with a demonstrated history of working in the hospital & private omfs. Web patient forms | uw medicine | patient enrollment section olumiant. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other.
For The Full List Of Excipients, See Section 6.1.
Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web scription and enrolment form will be kept on file and will not be reused. Web dedicated oral & maxillofacial surgeon with a demonstrated history of working in the hospital & private omfs. Web june 21, 2022.
Web Olumiant (Baricitinib) Is A Janus Kinase (Jak) Inhibitor Indicated For The Treatment Of Adult Patients With Moderately To Severely.
I certify that the use of olumiant® for this patient is. Adults with severe alopecia areata. Web olumiant® showed significant improvements in the severity and extent of atopic dermatitis and other. Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™.
Web Olumiant Can Be Used Either Alone Or In Combination With The Disease Modifying Drug Methotrexate;
Web alopecia areata, commonly referred to as just alopecia, is an autoimmune disorder in which the body attacks its own hair follicles,. Web new oral treatment for moderate to severe atopic dermatitis. Web olumiant (baricitinib)—a drug primarily used to treat rheumatoid arthritis and other autoimmune conditions—was. Web since i worked with xamarin.forms in the past, l wanted to give a shot to his big brother.
The Food And Drug Administration (Fda) Has Approved Olumiant (Baricitinib), A Janus Kinase.
Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Web patient enrollment section olumiant® (baricitinib) rheumatology office: I liked it… liked by seyed.