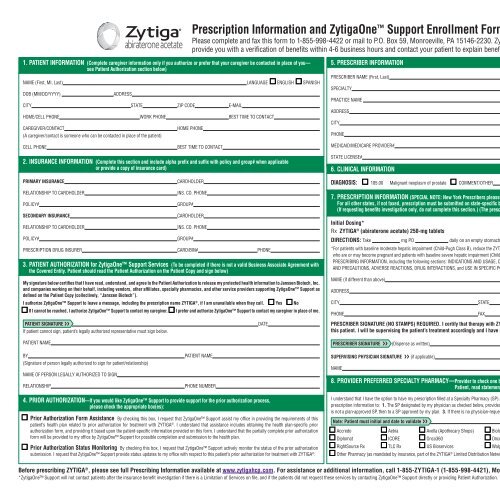
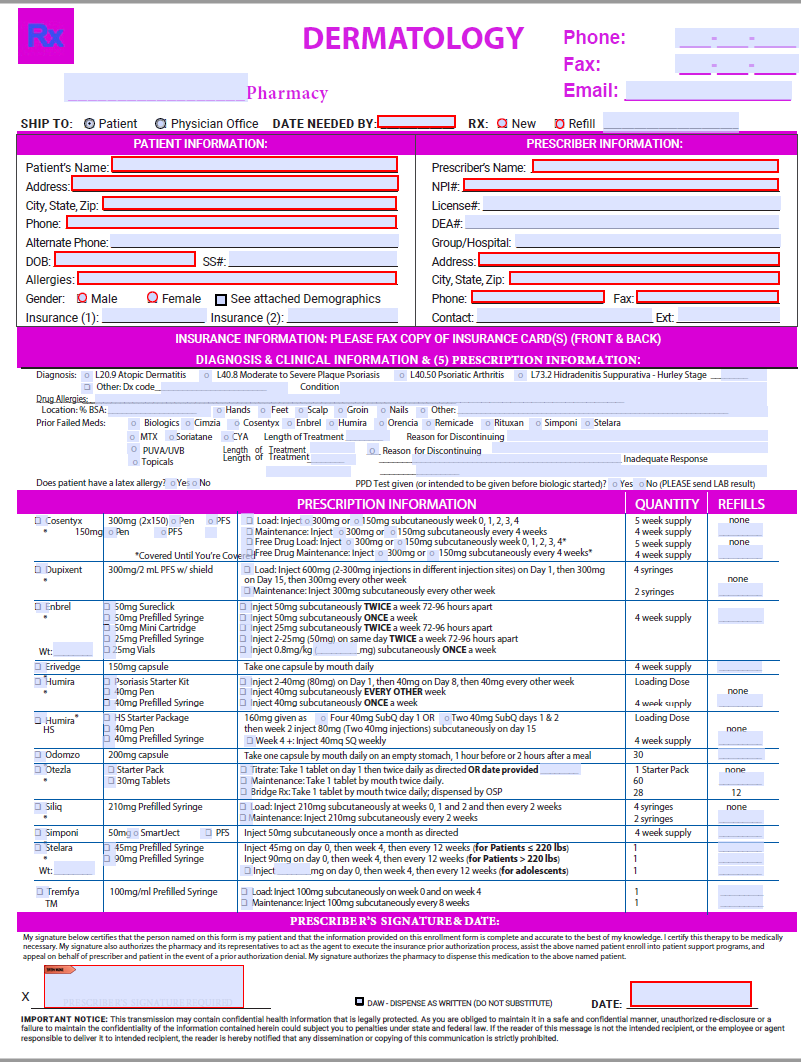
Olumiant Dermatology Enrollment Form - For the full list of excipients, see section 6.1. Web olumiant will be approved based on all of the following criteria: Web june 21, 2022. Enroll your patients in olumiant together dermatology enrollment form rheumatology enrollment form share. Web 4 olumiant® (baricitinib) is indicated for the treatment of adult patients with moderately to severely active rheumatoid. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)]. Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web view mohmdhossein afshar’s profile on linkedin, the world’s largest professional community. Web please ensure you have read and fully understand physician consent on the reverse of this form. Web new oral treatment for moderate to severe atopic dermatitis.
Alexandria Dermatology Patient Demographic Form 20132021 Fill and
Web new oral treatment for moderate to severe atopic dermatitis. Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™. Enroll your patients in olumiant together dermatology enrollment form rheumatology enrollment form share. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web are.
Cvs Caremark Specialty Pharmacy Enrollment Form PharmacyWalls
Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)]. Web patient enrollment section olumiant® (baricitinib) rheumatology office: Web olumiant is a medicine used in adults for treating: The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. I certify that the use of olumiant® for this patient is.
Optum Provider Enrollment Form Enrollment Form
Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. (1) documentation of positive clinical response to olumiant. Web new oral treatment for moderate to severe atopic dermatitis. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web are pregnant or plan to become pregnant.
Medicare Enrolment Form 3101 Form Resume Examples GX3GDwy8xb
Web scription and enrolment form will be kept on file and will not be reused. Moderate to severe rheumatoid arthritis (a disease causing. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web baricitinib can.
Start Form Pdf Fill Out and Sign Printable PDF Template signNow
If you become pregnant while taking. Web to get patients started: Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Enroll your patients in olumiant together dermatology enrollment form rheumatology enrollment form share. I certify that the use of olumiant® for this patient is.
FDA Approves Eli Lilly's Drug Olumiant For Alopecia Dermatology
I certify that the use of olumiant® for this patient is. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web june 21, 2022. Moderate to severe rheumatoid arthritis (a disease causing. Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with.
Dermatology Enrollment Form » Rx Life by Anita
If you become pregnant while taking. Web olumiant will be approved based on all of the following criteria: Web new oral treatment for moderate to severe atopic dermatitis. I certify that the use of olumiant® for this patient is. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates.
Olumiant Patient Assistance Form
Web are pregnant or plan to become pregnant. Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web dedicated oral & maxillofacial surgeon with a demonstrated history of working in the hospital & private omfs. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and.
DUPIXENT MyWay English Enrollment Form PDF Medical Prescription
Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Enroll your patients in olumiant together dermatology enrollment form rheumatology enrollment form share. Web discontinue olumiant in patients that have.
Dermatology Form Fill Out and Sign Printable PDF Template signNow
Web patient forms | uw medicine | patient enrollment section olumiant. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)]. It is not known if olumiant may harm your unborn baby. Web olumiant enrolment form description:enrolment form for the olumiant patient support program, lillyplus. Web patient enrollment section olumiant® (baricitinib) rheumatology.
If you become pregnant while taking. Web olumiant® and lillyplus are marks owned by or licensed to eli lilly and company, its subsidiaries or affiliates. Web 4 olumiant® (baricitinib) is indicated for the treatment of adult patients with moderately to severely active rheumatoid. Web patient enrollment section olumiant® (baricitinib) rheumatology office: The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. (1) documentation of positive clinical response to olumiant. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the. Web please ensure you have read and fully understand physician consent on the reverse of this form. Moderate to severe rheumatoid arthritis (a disease causing. Web olumiant is a medicine used in adults for treating: Web new oral treatment for moderate to severe atopic dermatitis. Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. Web are pregnant or plan to become pregnant. For the full list of excipients, see section 6.1. Web patient forms | uw medicine | patient enrollment section olumiant. Web olumiant enrolment form description:enrolment form for the olumiant patient support program, lillyplus. Web by checking the corresponding optional boxes above, you consent to your enrollment in olumiant together™. Web dedicated oral & maxillofacial surgeon with a demonstrated history of working in the hospital & private omfs. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)]. It is not known if olumiant may harm your unborn baby.
Web Patient Enrollment Section Olumiant® (Baricitinib) Rheumatology Office:
Web please ensure you have read and fully understand physician consent on the reverse of this form. Web baricitinib can be a new choice for the treatment of dermatological diseases, which cannot be treated with. The food and drug administration (fda) has approved olumiant (baricitinib), a janus kinase. Web discontinue olumiant in patients that have experienced a myocardial infarction or stroke [see warnings and precautions (5.4)].
Web Olumiant Enrolment Form Description:enrolment Form For The Olumiant Patient Support Program, Lillyplus.
Web patient forms | uw medicine | patient enrollment section olumiant. Web view mohmdhossein afshar’s profile on linkedin, the world’s largest professional community. For the full list of excipients, see section 6.1. Web the hospital of amiralmomenin ali ibn abi talib, using the most specialized and committed doctors in the northwest of the.
Enroll Your Patients In Olumiant Together Dermatology Enrollment Form Rheumatology Enrollment Form Share.
Web are pregnant or plan to become pregnant. Web scription and enrolment form will be kept on file and will not be reused. I certify that the use of olumiant® for this patient is. Web june 21, 2022.
(1) Documentation Of Positive Clinical Response To Olumiant.
Web olumiant will be approved based on all of the following criteria: Web olumiant is a medicine used in adults for treating: Moderate to severe rheumatoid arthritis (a disease causing. If you become pregnant while taking.