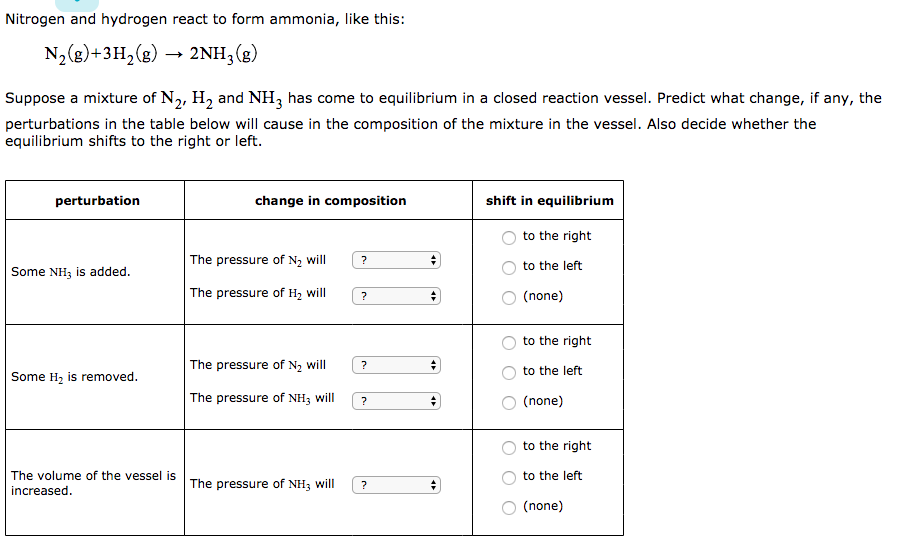
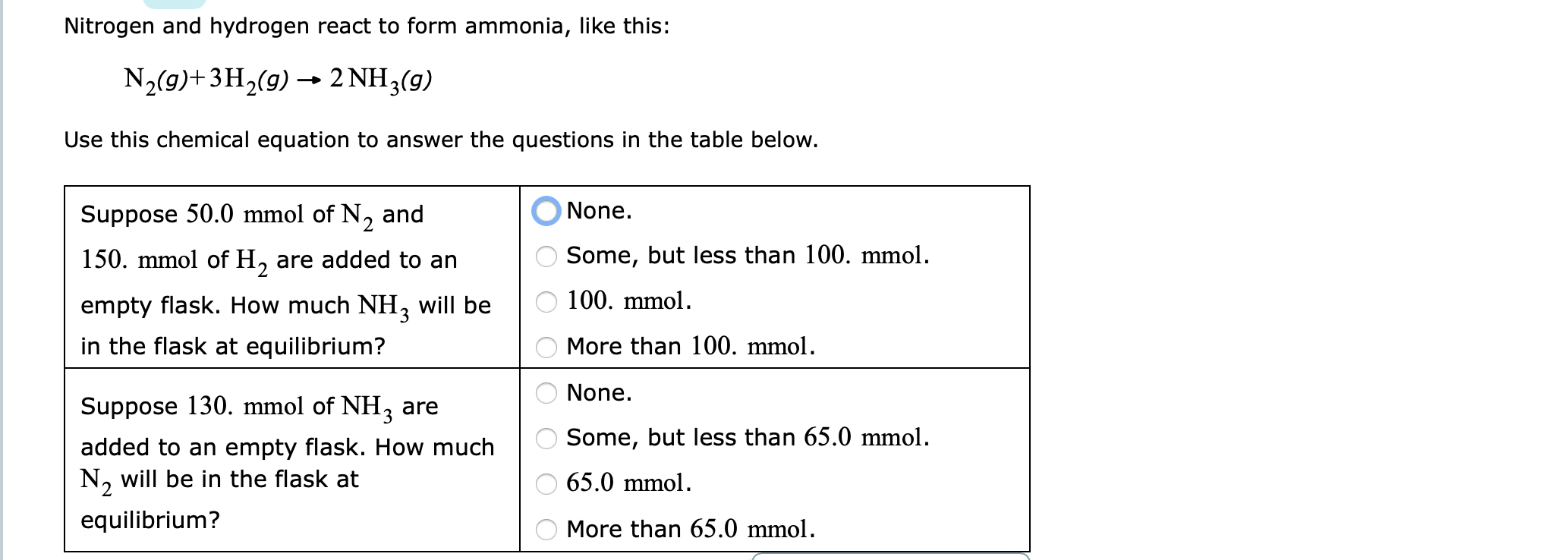
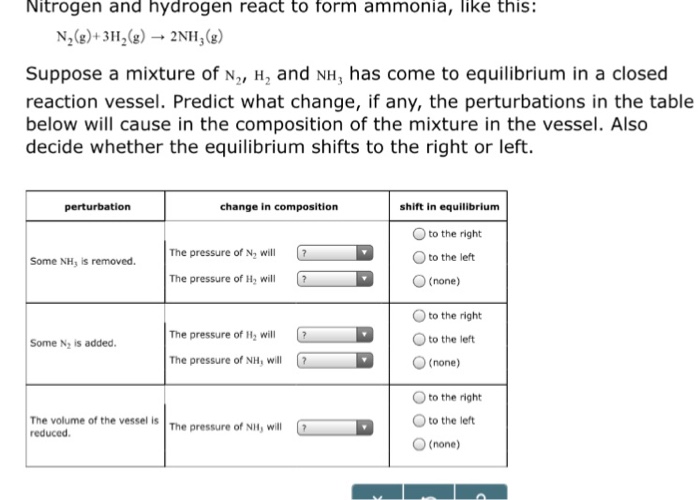
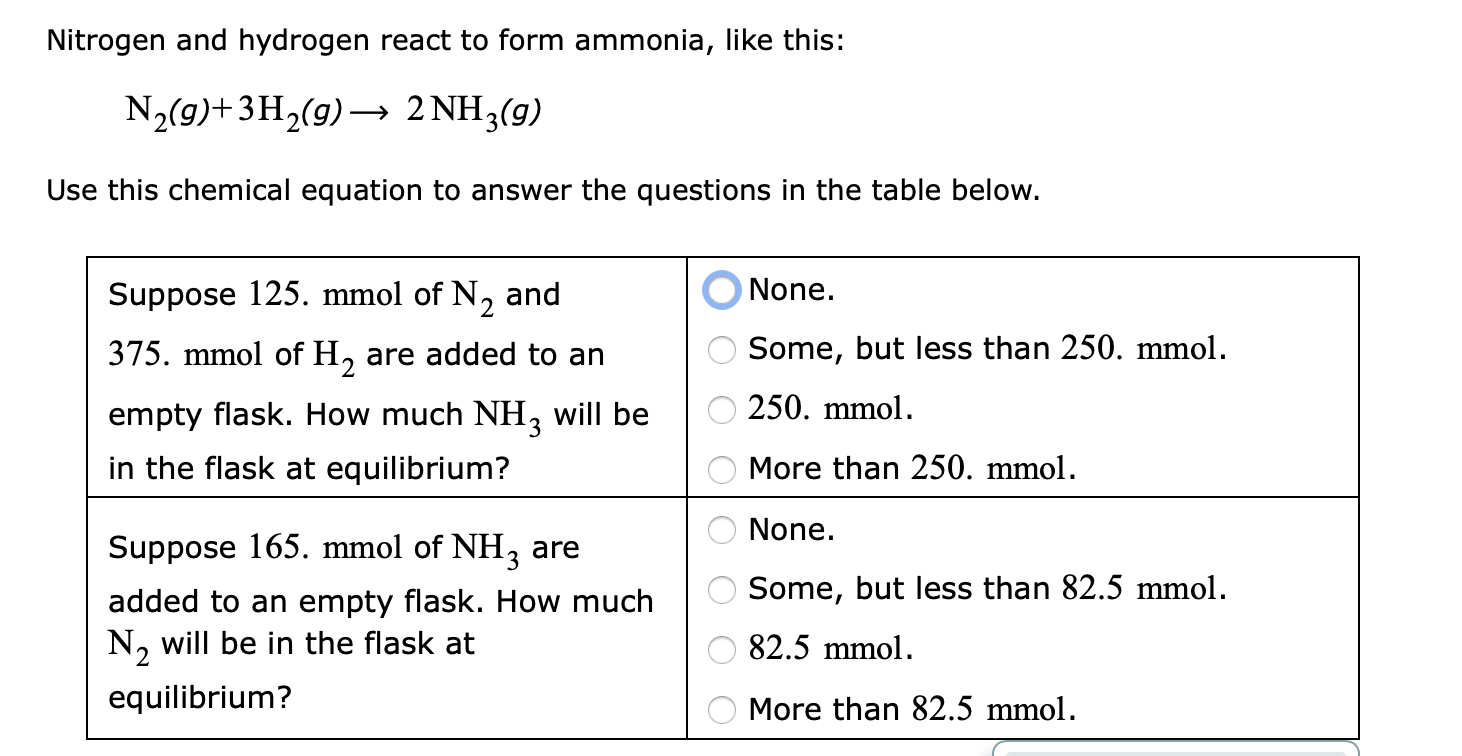
Nitrogen And Hydrogen React To Form Ammonia - At a certain temperature, nitrogen and hydrogen react to form ammonia: N2(g) + 3h2(g) ⎯→ 2nh3(g) the amount of ammonia that. Web we see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2 molecules of ammonia. Web nitrogen reacts with hydrogen to give ammonia. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Asked • 01/08/20 hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 +. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: N2 (g) + 3h2 (g) → 2nh3 (g).
Solved Nitrogen and hydrogen react to form ammonia, like
Web in an experiment, 2 g of nitrogen gas reacts with 6 g of hydrogen to form 8 g of ammonia. Web hydrogen reacts with nitrogen to form ammonia as: Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web hydrogen gas combines with nitrogen to form ammonia. Web nitrogen and hydrogen react to form ammonia.
Solved Nitrogen and hydrogen react to produce ammonia
Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Web hydrogen gas combines with nitrogen to form ammonia. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with 2.0. Web find the answer to the question about the limiting reactant of.
Solved Nitrogen and hydrogen react to form ammonia, like
Web nitrogen and hydrogen react to form ammonia, like this: N2(g) + 3h2(g) ⎯→ 2nh3(g) the amount of ammonia that. N2 (g) + 3h2 (g) → 2nh3 (g). Web the haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Web the web page provides a detailed solution to a chemistry question about.
Solved Nitrogen and hydrogen react to form ammonia, like
Web the haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. N2 (g) + 3h2 (g) → 2nh3 (g). Web hydrogen gas combines with nitrogen to form ammonia. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with.
Solved Nitrogen and hydrogen react to form ammonia, like
Web click here👆to get an answer to your question ️ nitrogen and hydrogen react to form ammonia as per the reaction 1/2n2 +. Web the web page provides a detailed solution to a chemistry question about the rate of the reverse reaction of nitrogen and hydrogen. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n.
Hydrogen reacts with nitrogen to produce ammonia according to this
Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of. Then when 2 g of nitrogen reacts with 12 g. Web nitrogen and hydrogen react to form ammonia, like this: Web click here👆to get an answer to your question ️ nitrogen and hydrogen react to form ammonia as per.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web fortunately, ammonia (nh 3) is emerging as a promising hydrogen carrier due to its high hydrogen content (17.6. Web nitrogen reacts with hydrogen to give ammonia. Asked • 01/08/20 hydrogen and nitrogen react to form ammonia according to.
Nitrogen and hydrogen react to form ammonia according to the equation
Web the haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web hydrogen and nitrogen react to form.
Solved Nitrogen and hydrogen react to form ammonia, like
Asked • 01/08/20 hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 +. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Web we see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2 molecules of ammonia. Web in these conditions, some of the hydrogen and.
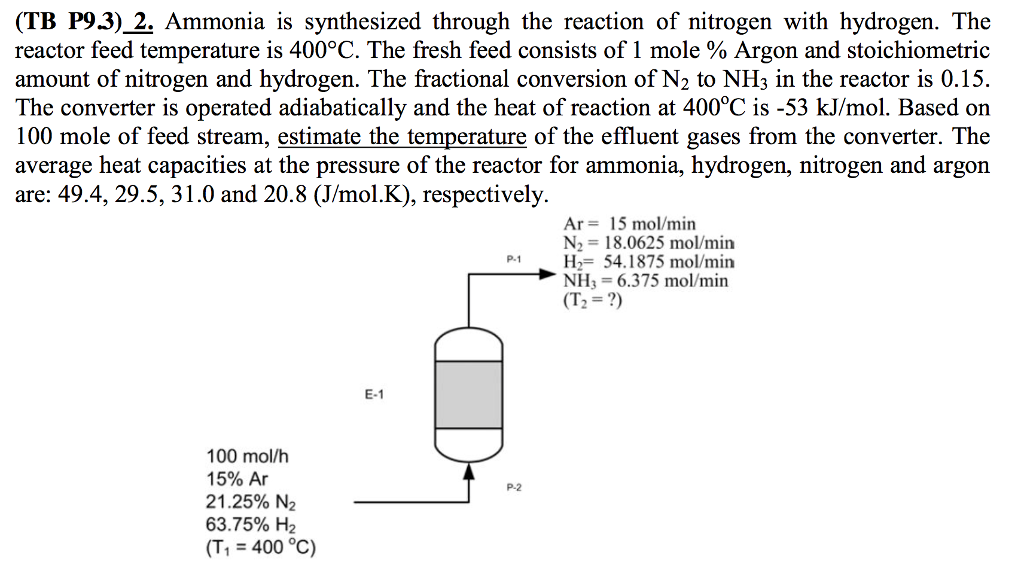
Solved Ammonia is synthesized through the reaction of
N2 (g) + 3h2 (g) → 2nh3 (g). Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Web nitrogen and hydrogen react to form ammonia, like this: At a certain temperature, nitrogen and hydrogen react to form ammonia: Web we see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2.
Web find the answer to the question about the limiting reactant of ammonia formation from nitrogen and hydrogen in different starting. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen and hydrogen react to form ammonia, like this: Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a. N2(g) + 3h2(g) ⎯→ 2nh3(g) the amount of ammonia that. Then when 2 g of nitrogen reacts with 12 g. Calculate the volume of the ammonia gas formed when nitrogen reacts. Web hydrogen gas combines with nitrogen to form ammonia. At a certain temperature, nitrogen and hydrogen react to form ammonia: Web the web page provides a detailed solution to a chemistry question about the rate of the reverse reaction of nitrogen and hydrogen. Web click here👆to get an answer to your question ️ nitrogen and hydrogen react to form ammonia as per the reaction 1/2n2 +. Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with 2.0. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of. Web fortunately, ammonia (nh 3) is emerging as a promising hydrogen carrier due to its high hydrogen content (17.6. N2 (g) + 3 h2 (g) =. Yes, it is absolutely ture, when hydrogen react with. Web chemistry questions and answers. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. N2 (g) + 3h2 (g) → 2nh3 (g). N2 (g) + 3h2 (g) → 2nh3 (g).
Web Click Here👆To Get An Answer To Your Question ️ Nitrogen And Hydrogen React To Form Ammonia As Per The Reaction 1/2N2 +.
Web we see that 1 molecule of nitrogen reacts with 3 molecules of hydrogen to form 2 molecules of ammonia. Then when 2 g of nitrogen reacts with 12 g. At a certain temperature, nitrogen and hydrogen react to form ammonia: Web the web page provides a detailed solution to a chemistry question about the rate of the reverse reaction of nitrogen and hydrogen.
Web Fortunately, Ammonia (Nh 3) Is Emerging As A Promising Hydrogen Carrier Due To Its High Hydrogen Content (17.6.
N2 (g) + 3h2 (g) rightarrow 2nh3 (g) use. Yes, it is absolutely ture, when hydrogen react with. Web nitrogen and hydrogen react to form ammonia, like this: Web hydrogen and nitrogen react to form ammonia according to the reaction, 3 h2 + n2 2 nh3 if 4.0 moles of h2 with 2.0.
N2 (G) + 3 H2 (G) =.
N2(g) + 3h2(g) ⎯→ 2nh3(g) the amount of ammonia that. Web nitrogen and hydrogen react to form ammonia according to the following balanced equation: Web in these conditions, some of the hydrogen and nitrogen will react to form ammonia. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a.
N2 (G) + 3H2 (G) → 2Nh3 (G).
Web chemistry questions and answers. Web according to the stoichiometric coefficients, 1 mol of nitrogen reacts with 3 moles of hydrogen to form 2 moles of. Web the haber process combines nitrogen from the air with hydrogen derived mainly from natural gas (methane) into ammonia. Web hydrogen gas combines with nitrogen to form ammonia.