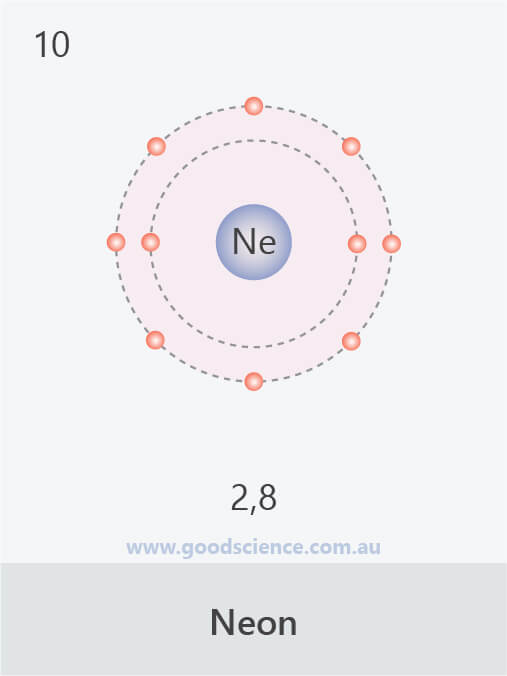
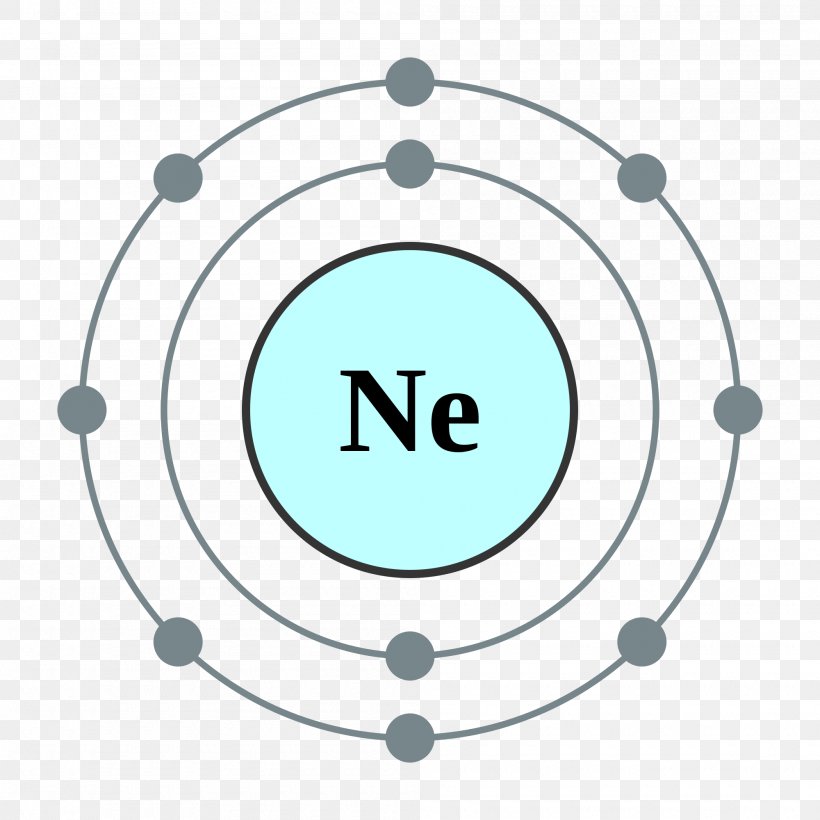
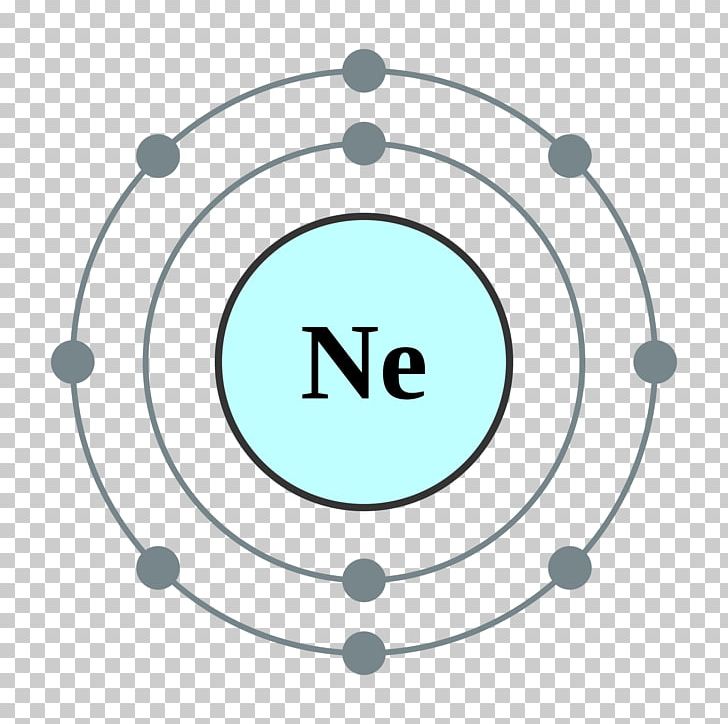
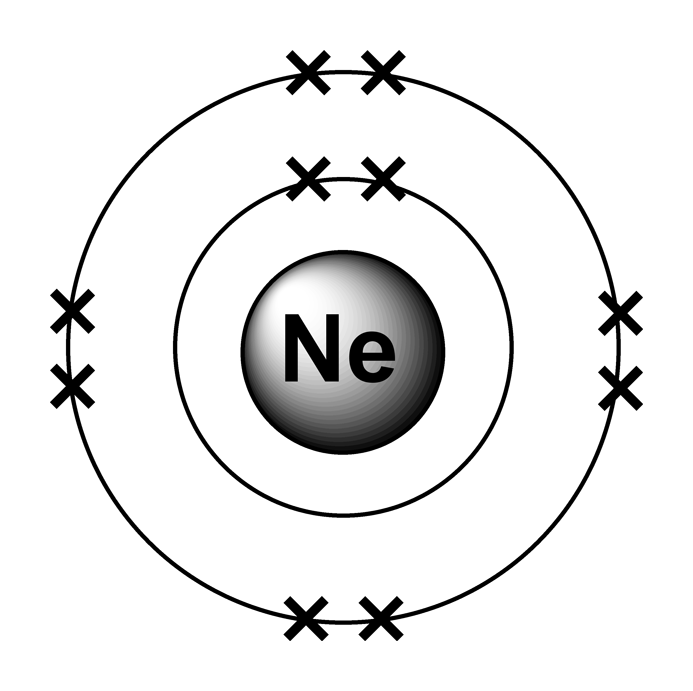
Neon Electron Configuration Long Form - All of the electrons in the noble gas neon. For each atom the subshells are. Since it is the outermost. Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Web 111 rows the commonly used long form of the periodic table is designed to emphasize electron configurations. The electronic configuration of neon is ne: Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. The total electrical charge of the nucleus is therefore +ze, where e (elementary. Web the complete electron configuration of neon is 1s 2 2s 2 2p 6.neon has 8 valence electrons around the nucleus and the. Web 1 answer doc croc.
Abbreviated Electron Configuration For Neon Rapid Electron
1s 2 2s 2 2p 6. The first ten electrons of the. A noble gas configuration of an atom consists of the elemental symbol of. Web the nucleus is composed of. You can also study its electron.
Neon Element (Ne 10) of Periodic Table Periodic Table FlashCard
Web electron configuration the arrangements of electrons above the last (closed shell) noble gas. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Melting point the temperature at. Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. Web iron has 26 electrons so its.
[5 Steps] Electron Configuration for or of Neon in Just 5 Steps
Web we have this configuration number as [he] 2s2 2p6 for the chemical element. May 31, 2014 [2,8] or 1s22s22p6 neon has atomic number 10, so a neon atom has 10 protons. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its. Web 1 answer doc croc. In writing the electron configuration for.
Electron Configuration (Elements 120) Good Science
Web we have this configuration number as [he] 2s2 2p6 for the chemical element. The electronic configuration of neon is ne: All of the electrons in the noble gas neon. Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the. 1s 2 2s 2 2p 6.
Electron Configuration for Neon (Ne) Full Explanation
Web electron configuration of boron (b) [he] 2s 2 2p 1: Possible oxidation states are 0. A noble gas configuration of an atom consists of the elemental symbol of. Web 111 rows the commonly used long form of the periodic table is designed to emphasize electron configurations. The total electrical charge of the nucleus is therefore +ze, where e (elementary.
Neon Electron Configuration Noble Gas Valence Electron Lewis Structure
Fe 1s22s22p63s23p64s23d6 when we make a 3+ ion for iron, we need to take the. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states. Web the abbreviated electronic configuration of neon is [he] 2s2 2p6. All of the electrons in the noble gas neon. A noble gas configuration of an atom consists of.
Electron Configuration Of Neon Long Form worksheet today
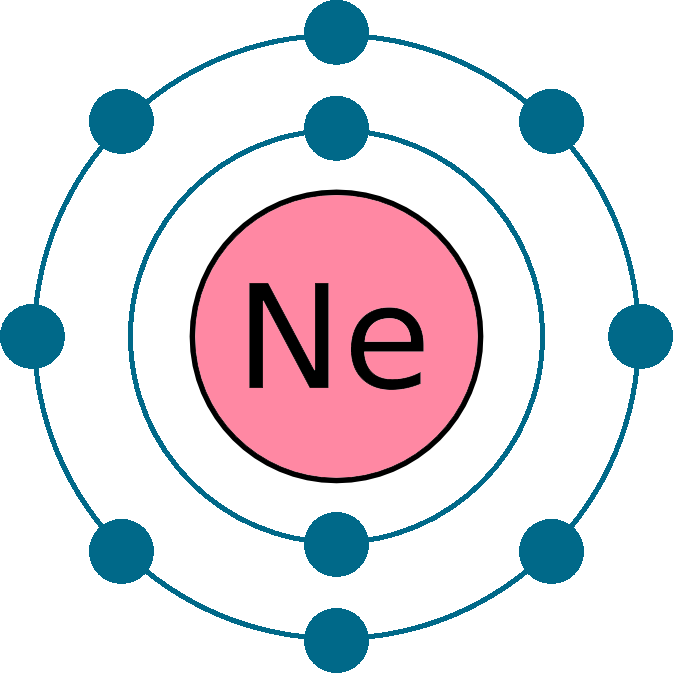
Web electron configuration of neon is [he] 2s2 2p6. The first ten electrons of the. Web therefore, the electron configuration of lithium is: Electronic configuration of neon = 2, 8.neon is a chemical element with symbol ne and atomic number 10. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its.
Neon Electron Configuration Noble Gas Valence Electron Lewis Structure
1s 2 2s 2 2p 1: You can also study its electron. Web neon is the tenth element with a total of 10 electrons. It is in group 18. Boron (atomic number 5) has five electrons.
How many valence electrons does neon(Ne) have?
Boron (atomic number 5) has five electrons. Web they are helium, neon, argon, krypton, xenon, and radon. To do electron configuration of neon,we have to know the atomic number of neon. All of the electrons in the noble gas neon. Web we have this configuration number as [he] 2s2 2p6 for the chemical element.
Electron configurations
To form abbreviated notation of electronic configuration, the. All of the electrons in the noble gas neon. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its. It is in group 18. The electronic configuration of neon is ne:
Web the complete electron configuration of neon is 1s 2 2s 2 2p 6.neon has 8 valence electrons around the nucleus and the. Web electron configuration of neon is [he] 2s2 2p6. All of the electrons in the noble gas neon. A noble gas configuration of an atom consists of the elemental symbol of. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). 1s 2 2s 2 2p 1: Boron (atomic number 5) has five electrons. Web electron configuration of boron (b) [he] 2s 2 2p 1: Web therefore, the electron configuration of lithium is: Electron configuration of carbon (c) [he] 2s 2 2p 2: Web 1 answer doc croc. Web we have this configuration number as [he] 2s2 2p6 for the chemical element. Possible oxidation states are 0. To do electron configuration of neon,we have to know the atomic number of neon. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its. 1s 2 2s 2 2p 6. To form abbreviated notation of electronic configuration, the. Web the arrangement of electrons in the orbitals of an atom is called the electron configuration of the atom. Web neon is the tenth element with a total of 10 electrons. Web the abbreviated electronic configuration of neon is [he] 2s2 2p6.
Web The Abbreviated Electronic Configuration Of Neon Is [He] 2S2 2P6.
A noble gas configuration of an atom consists of the elemental symbol of. The electron configuration of sodium is \(1s^2 2s^2 2p^6 3s^1\) (table \(\pageindex{1}\)). All of the electrons in the noble gas neon. The electronic configuration of neon is ne:
To Form Abbreviated Notation Of Electronic Configuration, The.
To do electron configuration of neon,we have to know the atomic number of neon. Melting point the temperature at. Web 1 answer doc croc. Web we have this configuration number as [he] 2s2 2p6 for the chemical element.
You Can Also Study Its Electron.
Possible oxidation states are 0. The arrangement of electrons into the orbitals of an atom using some fundamental principle is called its. Web 111 rows the commonly used long form of the periodic table is designed to emphasize electron configurations. For each atom the subshells are.
1S 2 2S 2 2P 6.
Web fluorine (atomic number 9) has only one 2 p orbital containing an unpaired electron. 1s 2 2s 2 2p 1: May 31, 2014 [2,8] or 1s22s22p6 neon has atomic number 10, so a neon atom has 10 protons. Web this page shows the electron configurations of the neutral gaseous atoms in their ground states.


![[5 Steps] Electron Configuration for or of Neon in Just 5 Steps](https://2.bp.blogspot.com/-jMs9FCVMeSw/XD4FtZiSVlI/AAAAAAAAYZg/rJnlLKM6S6EHv8FmJZr4pj2PmzTZjk0hQCLcBGAs/s1600/20190115_220744.jpg)