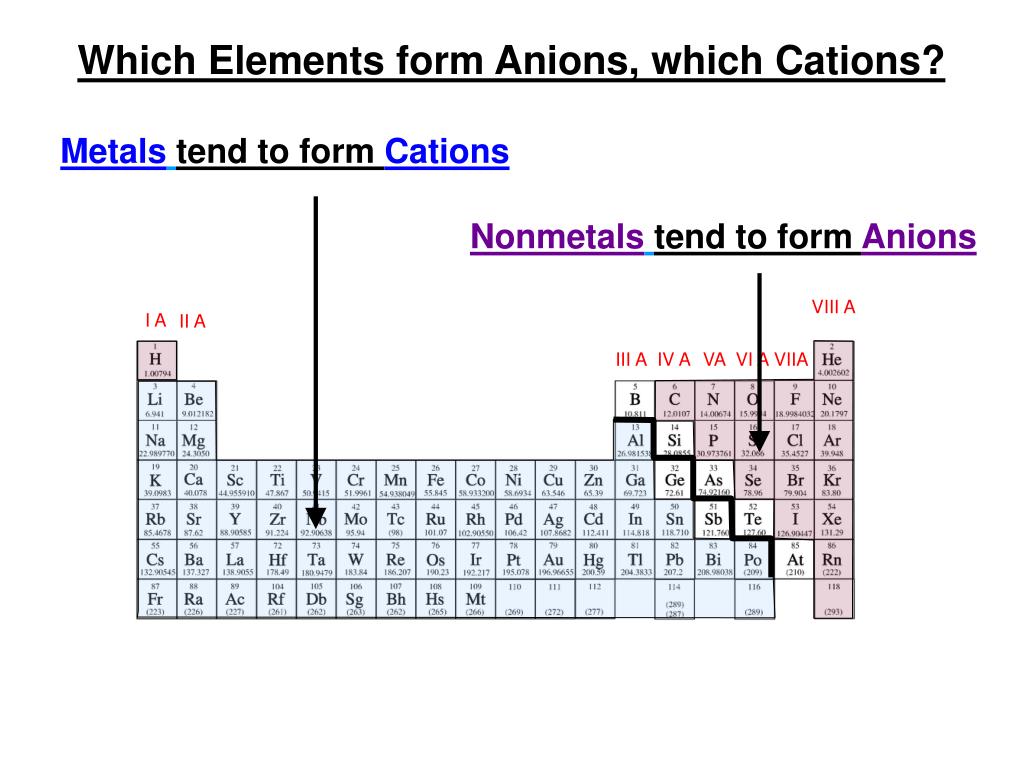
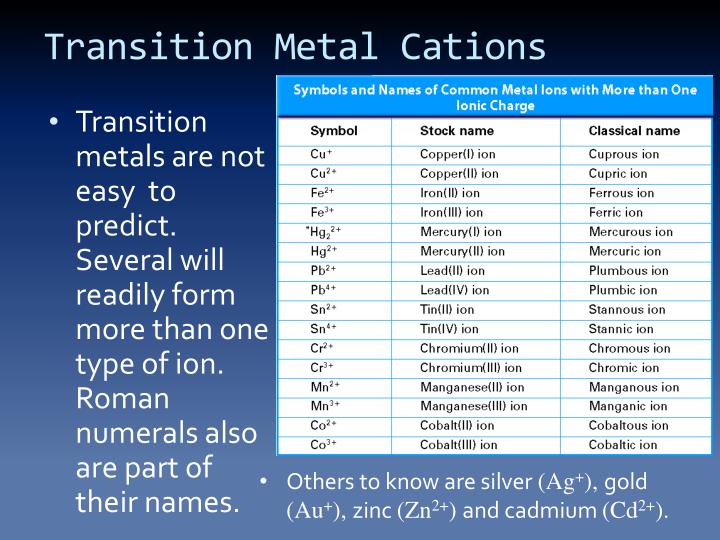
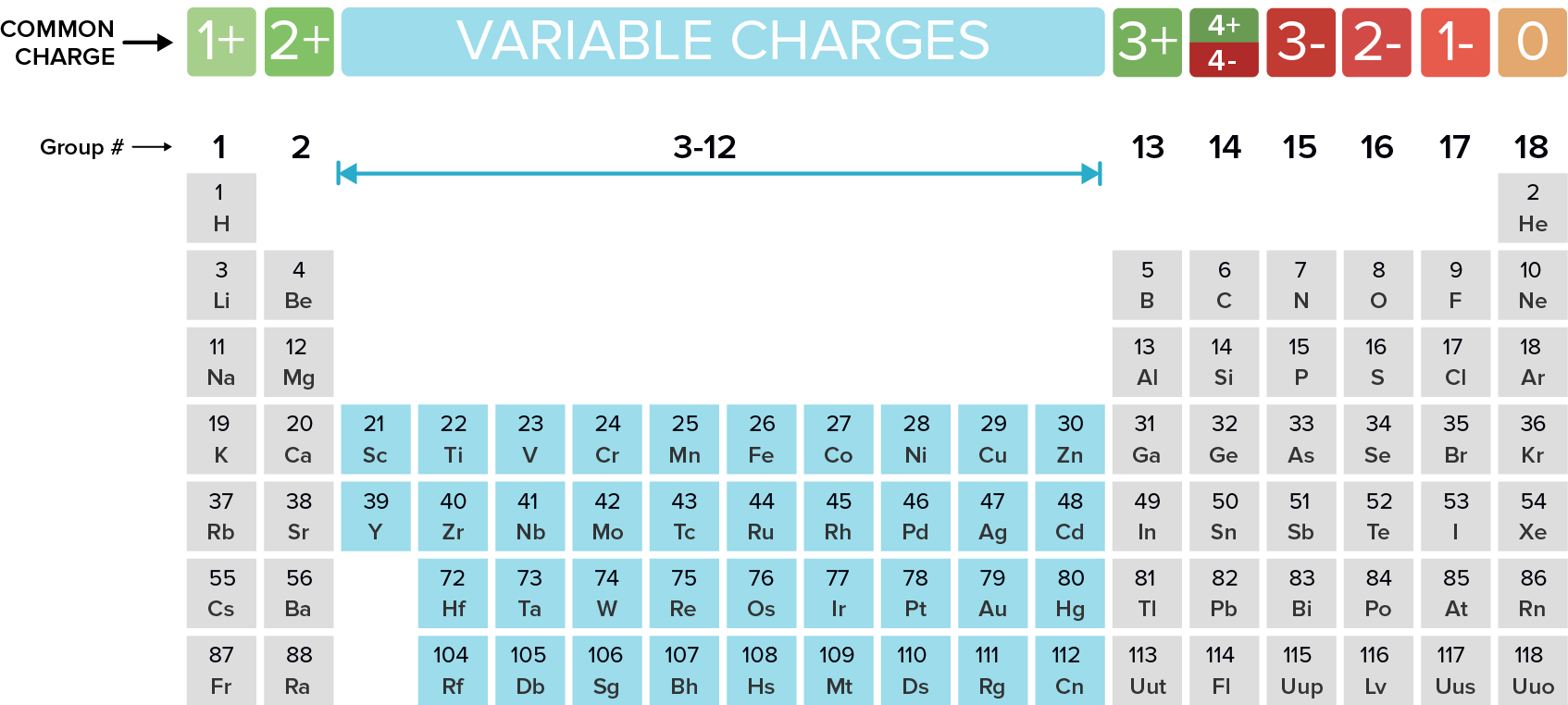
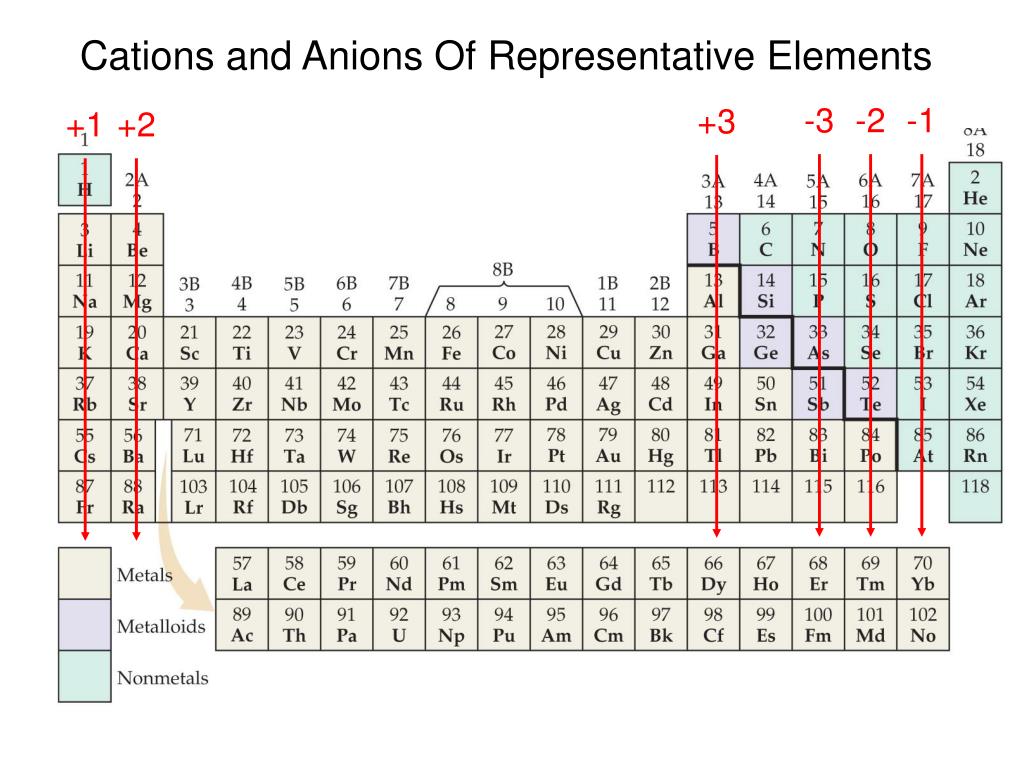
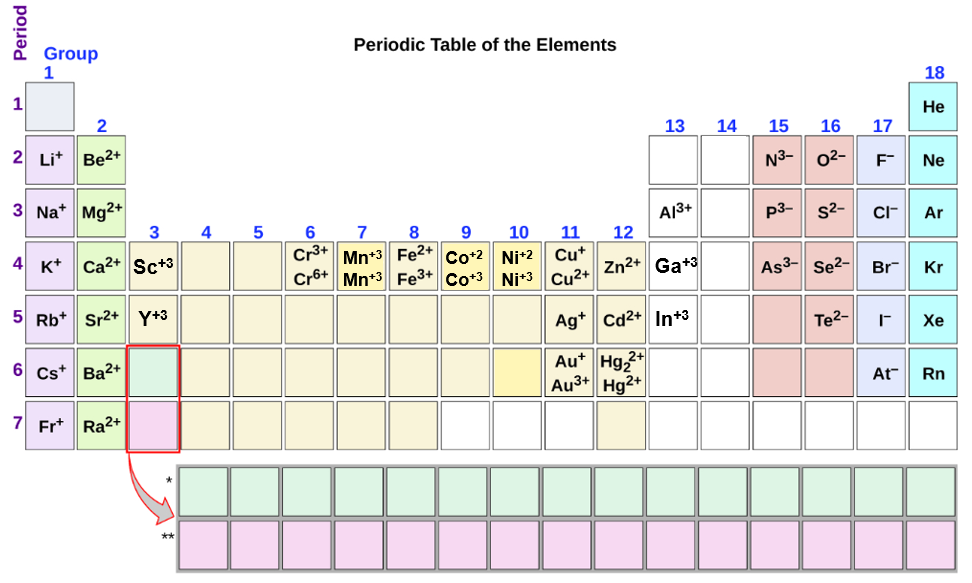
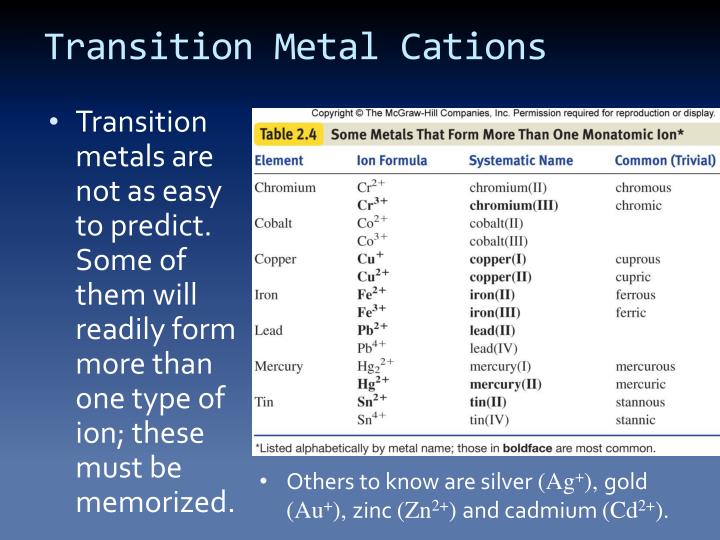
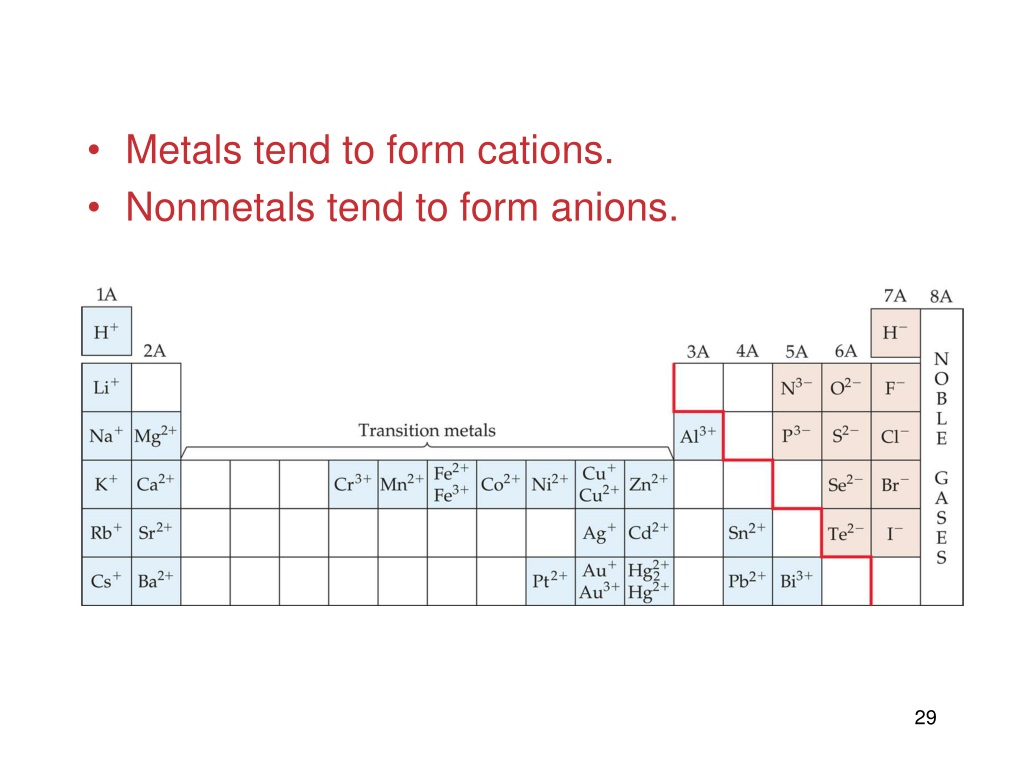
Metals Tend To Form Cations - Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web generally, metals tend to lose electrons to form cations. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are. For example, the alkali metals and the alkaline earth metals. Web answer (1 of 2): Web in this study, the pyridine and phenanthroline diphosphonate ligands were investigated for the first time in the context of solvent. Web cations are positively charged ions, meaning they have lost electrons and have a net positive charge.anions, on the. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Metals lose electrons in bonding, so they form cations, which are positively charged.
PPT Lecture 4. Chapter 2. Structure of the Atom (Contd.) PowerPoint
Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are. Web metallic atoms hold some of their electrons relatively loosely. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble.
PPT Naming IONS & formulas for Ionic Compounds PowerPoint
Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Most metal oxides are basic oxides and dissolve in water to form. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web even if the the metal has a smaller atomic radius.
Periodic Table Labeled With Groups And Charges Matttroy
Web generally, metals tend to lose electrons to form cations. Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Web so far, we have considered.
PPT Mastering Chemistry PowerPoint Presentation, free download ID
Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. Ii and iv explaination : Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity. Web expert answer 100% (8 ratings) correct option.
PPT 1 Name the ions formed by these elements and classify them as
Nonmetals tend to gain electrons to form anions. Metals lose electrons in bonding, so they form cations, which are positively charged. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity. Web cations are atoms that contain a positive charge, and they are formed when.
Do You Know How to Tell Cation and Anion Ions Apart? Chemistry
Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. Metals lose electrons in bonding, so they form cations, which are positively charged. For example, the alkali metals and the alkaline earth metals. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Nonmetals tend to gain electrons to form.
PPT Ionic Bonding PowerPoint Presentation, free download ID5679234
Nonmetals tend to gain electrons to form anions. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity. Most metal oxides are.
4.3 Ionic Compounds and Formulas (2022)
Web answer (1 of 2): Hence, te correct option is a. Consequently, they tend to lose electrons and form cations. For example, the alkali metals and the alkaline earth metals. Web science chemistry metals tend to form cations and almost have positive oxidation numbers a.
PPT IONS PowerPoint Presentation ID2435906
Web expert answer 100% (8 ratings) correct option : Web metallic atoms hold some of their electrons relatively loosely. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web generally, metals tend to lose electrons to form cations. Web transition metals achieve stability by arranging their.
PPT Chapter 7 Periodic Properties of the Elements PowerPoint
Most metal oxides are basic oxides and dissolve in water to form. Web metals tend to lose electrons and form positively charged ions called cations. Web generally, metals tend to lose electrons to form cations. Web even if the the metal has a smaller atomic radius due to occupying less shells, the affinity for the electrons from. For metals, the.
Consequently, they tend to lose electrons and form cations. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. For example, the alkali metals and the alkaline earth metals. Web generally, metals tend to lose electrons to form cations. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are. Web hence, metals tends to form cations. Web metals tend to lose electrons and form positively charged ions called cations. Most metal oxides are basic oxides and dissolve in water to form. Hence, te correct option is a. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Web in this study, the pyridine and phenanthroline diphosphonate ligands were investigated for the first time in the context of solvent. Ii and iv explaination : Web cations are positively charged ions, meaning they have lost electrons and have a net positive charge.anions, on the. Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Nonmetals tend to gain electrons to form anions. Web answer (1 of 2): Web science chemistry metals tend to form cations and almost have positive oxidation numbers a. Web so far, we have considered elements that typically form cations of one particular charge. Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal.
Web Hence, Metals Tends To Form Cations.
For metals, the highest (outermost) energy. Web metals have several qualities that are unique, such as the ability to conduct electricity and heat, a low ionization energy, and a low electronegativity. Web first, metals tend to from cations and nonmetals tend to form anions, while the noble gasses do not tend to form ions. Web answer (1 of 2):
Web So Far, We Have Considered Elements That Typically Form Cations Of One Particular Charge.
Web first, each element that forms cations is a metal, except for one (hydrogen), while each element that forms anions is a nonmetal. Web solved:explain why metals tend to form cations, while nonmetals tend to form anions. Web metallic atoms hold some of their electrons relatively loosely. Web metals are electropositive elements that generally form basic or amphoteric oxides with oxygen.
Web Expert Answer 100% (8 Ratings) Correct Option :
Metals lose electrons in bonding, so they form cations, which are positively charged. Consequently, they tend to lose electrons and form cations. For example, the alkali metals and the alkaline earth metals. Web cations are atoms that contain a positive charge, and they are formed when the atoms lose electrons which are.
Nonmetals Tend To Gain Electrons To Form Anions.
Web transition metals achieve stability by arranging their electrons accordingly and are oxidized, or they lose. Web in this study, the pyridine and phenanthroline diphosphonate ligands were investigated for the first time in the context of solvent. Web generally, metals tend to lose electrons to form cations. Most metal oxides are basic oxides and dissolve in water to form.