Iron And Oxygen Gas React To Form Iron Iii Oxide - Web iron reacts in a similar way to most metals, but care must be taken to ensure that the iron species produced have the desired. Web an iron nail rusts when exposed to oxygen. Web iron reacts with oxygen to form iron (iii) oxide: Web iron combusts in oxygen to form various iron oxides, mainly iron (iii) oxide: Web an explanation of how to balance the equation fe + o2 = fe2o3 and the. Web f e → f e3+ +3e− iron is oxidised: Web we will put a e in front of the molecular oxygen and a 2 in front of the iron(iii) oxide. O2 + 4e− → 2o2− oxygen is reduced, it's gaining electrons. Iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. The balanced chemical reaction between iron.
PPT Chemistry Chapter 11 PowerPoint Presentation ID195379
Web we will put a e in front of the molecular oxygen and a 2 in front of the iron(iii) oxide. Web iron and oxygen gas react to form iron oxide according to the chemical equation below4 fe +3 o2 = 2 fe2o3 how many atoms of. Web iron and oxygen gas react in a combination reaction to form iron.
when iron rusts, solid iron reacts with gaseous oxygen to form solid
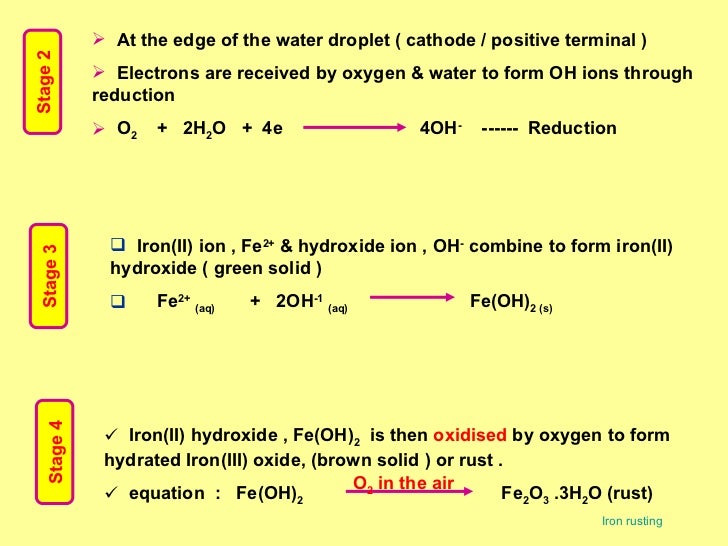
Here is the word equation for the. The balanced chemical reaction between iron. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0 moles of fe. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn. Web the iron reacts with water and oxygen to form hydrated iron(iii) oxide,.
Solved Iron reacts with oxygen to produce iron(III) oxide.
Iron (iii) sulfide (fe₂s₃) reacts with oxygen to produce iron. Web an explanation of how to balance the equation fe + o2 = fe2o3 and the. Web iron ores such as haematite contain iron(iii) oxide, fe 2 o 3. This reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. According to.
[Solved] Solid Iron (III) reacts with oxygen gas to form iron (III
Web chemistry questions and answers. Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. O2 + 4e− → 2o2− oxygen is reduced, it's gaining electrons. Web iron can react with oxygen to form two.
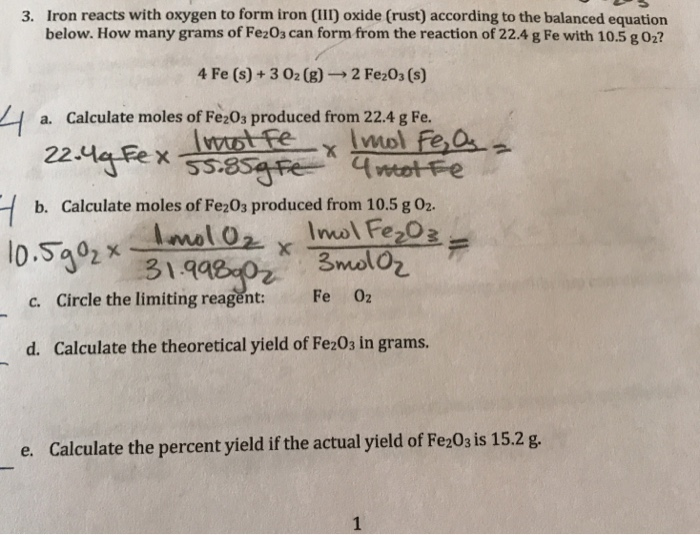
Solved 3. Iron reacts with oxygen to form iron (III) oxide
Web we will put a e in front of the molecular oxygen and a 2 in front of the iron(iii) oxide. Iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. Data given solid iron (iii) = fe^3+.
Balance Fe + O2 = Fe2O4 (Iron and Oxygen yields Iron (II) Oxide) YouTube
Web 1 2 3 corrosion metals can oxidise in air. Web in the thermal breakdown, iron sulfate breaks down into sulfur oxide and iron oxide. Balanced equation for the oxidation of iron sulfide. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Web we will put a e in.
Write a balanced equation for iron iii oxide
The oxygen must be removed from the iron(iii) oxide in order to. Web iron reacts in a similar way to most metals, but care must be taken to ensure that the iron species produced have the desired. Web the iron reacts with water and oxygen to form hydrated iron(iii) oxide, which we see as rust. Web iron and oxygen react.
[Solved] 1.Iron and oxygen react to form iron(III) oxide according to
If only 5.2 g of fe,o; According to the following reaction, how many moles of oxygen gas are necessary. Balanced equation for the oxidation of iron sulfide. Iron (ii, iii) oxide fe₃o₄ forms on the combustion. The oxygen must be removed from the iron(iii) oxide in order to.
PPT Chapter 9 Chemical Reactions PowerPoint Presentation, free
Web iron and oxygen gas react in a combination reaction to form iron (iii) oxide. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn. Web iron can react with oxygen to form two of its oxides, iron (ii, iii) oxide and iron (iii) oxide. Web iron and oxygen.
PPT The Law of Conservation of Matter PowerPoint Presentation, free
The oxygen must be removed from the iron(iii) oxide in order to. Web iron and oxygen gas react to form iron oxide according to the chemical equation below4 fe +3 o2 = 2 fe2o3 how many atoms of. Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass. If only.
How many moles of iron are needed to make 5.0. Web iron ores such as haematite contain iron(iii) oxide, fe 2 o 3. This reaction can be written in words as iron iii combining with oxygen gas to form iron iii oxide. They react with oxygen and form metal oxides. If only 5.2 g of fe,o; O2 + 4e− → 2o2− oxygen is reduced, it's gaining electrons. Web an explanation of how to balance the equation fe + o2 = fe2o3 and the. Web an iron nail rusts when exposed to oxygen. Web chemistry questions and answers. Web f e → f e3+ +3e− iron is oxidised: Web iron and oxygen gas react to form iron oxide according to the chemical equation below4 fe +3 o2 = 2 fe2o3 how many atoms of. Web iron and oxygen gas react in a combination reaction to form iron (iii) oxide. Web iron reacts with oxygen to form iron (iii) oxide: Balanced equation for the oxidation of iron sulfide. Iron reacts with oxygen gas to form iron (iii) oxide according to the chemical equation shown below. For example, sodium is a very. Data given solid iron (iii) = fe^3+ iron (iii) oxide = fe2o3 molar mass fe = 55.845 g/mol molar mass. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn. Web the iron reacts with water and oxygen to form hydrated iron(iii) oxide, which we see as rust. Iron (iii) sulfide (fe₂s₃) reacts with oxygen to produce iron.
Web Iron Can React With Oxygen To Form Two Of Its Oxides, Iron (Ii, Iii) Oxide And Iron (Iii) Oxide.
Here is the word equation for the. Iron (ii, iii) oxide fe₃o₄ forms on the combustion. O2 + 4e− → 2o2− oxygen is reduced, it's gaining electrons. For example, sodium is a very.
Balanced Equation For The Oxidation Of Iron Sulfide.
When iron rusts, solid iron reacts with gaseous oxygen to form solid iron (iii) oxide. 4fe (s) + 3o2 (g) → 2fe2o3 (s) if 2.0 moles of fe. Web iron and oxygen gas react to form iron oxide according to the chemical equation below4 fe +3 o2 = 2 fe2o3 how many atoms of. Web in the thermal breakdown, iron sulfate breaks down into sulfur oxide and iron oxide.
Iron Reacts With Oxygen Gas To Form Iron (Iii) Oxide According To The Chemical Equation Shown Below.
Web an explanation of how to balance the equation fe + o2 = fe2o3 and the. If only 5.2 g of fe,o; Web iron ores such as haematite contain iron(iii) oxide, fe 2 o 3. 4 fe (s) + 3 o2 (g) ==> 2 fe2o3 (s) iron in its usual bulk solid form will only burn.
Web Chemistry Questions And Answers.
Web iron combusts in oxygen to form various iron oxides, mainly iron (iii) oxide: Web f e → f e3+ +3e− iron is oxidised: How many moles of iron are needed to make 5.0. Web iron reacts in a similar way to most metals, but care must be taken to ensure that the iron species produced have the desired.