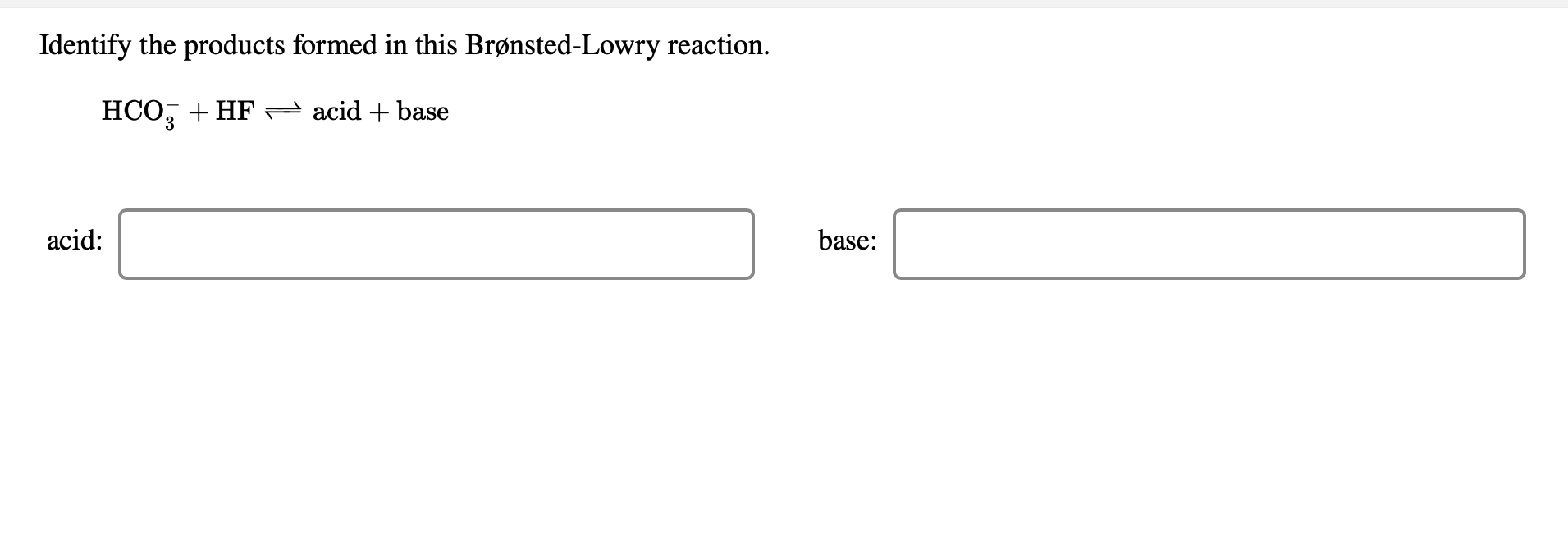
Identify The Products Formed In This Brønsted-Lowry Reaction. - Hpo2 + bro = acid + base acid: So2 hf h2o h2so4 albr3. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Dissolving an acid in water to form the hydronium ion and the anion of the acid. Web about this tutor ›. Hco +bro acid +base acid: Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Hco3 + f = acid. Web science chemistry chemistry questions and answers 1.
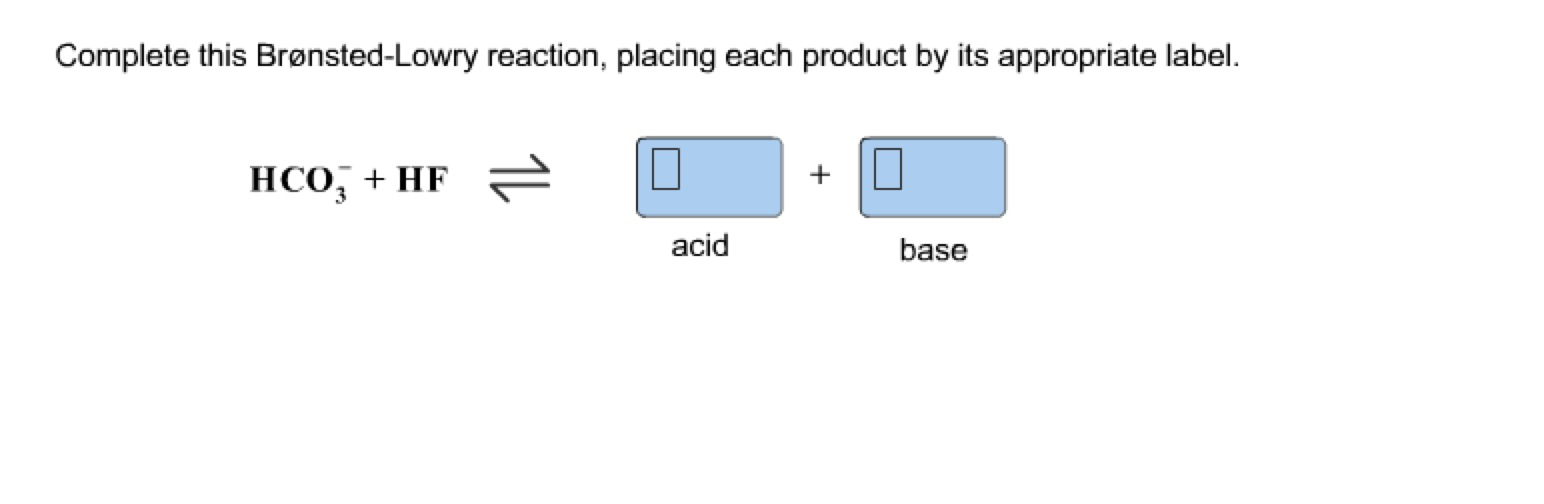
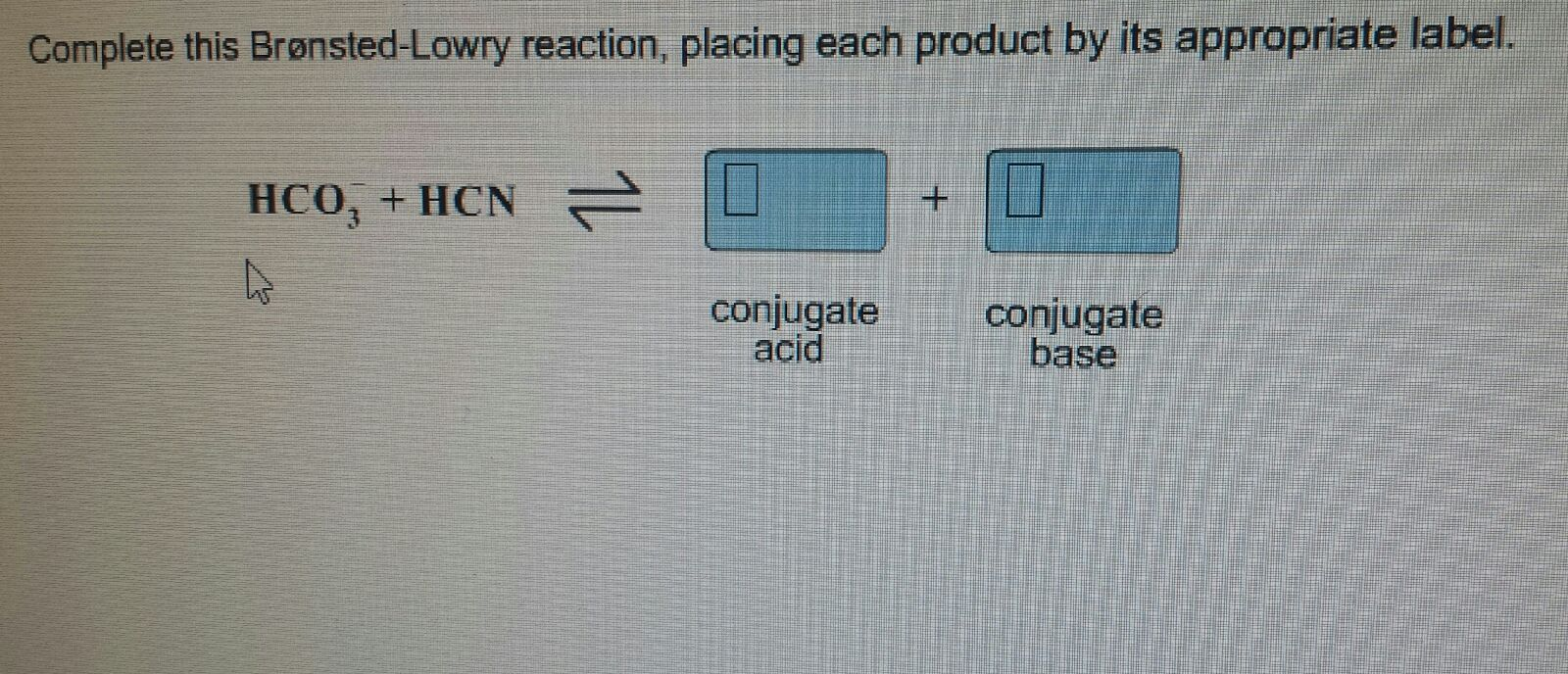
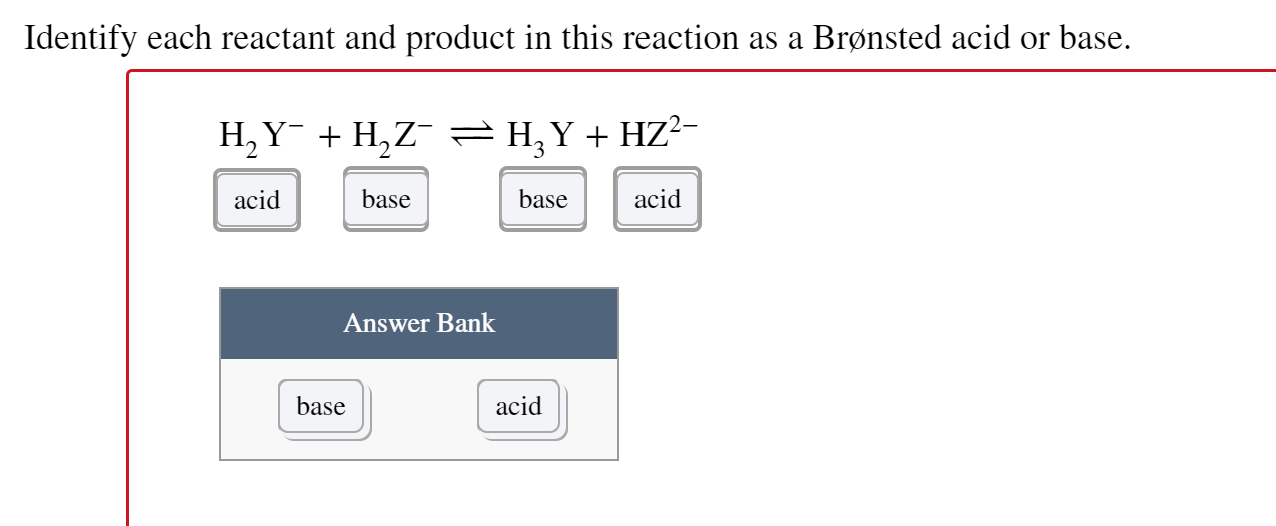
Solved Complete This BronstedLowry Reaction, Placing Eac...
Web about this tutor ›. So2 hf h2o h2so4 albr3. Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Hco3 + f = acid. Hco +bro acid +base acid:
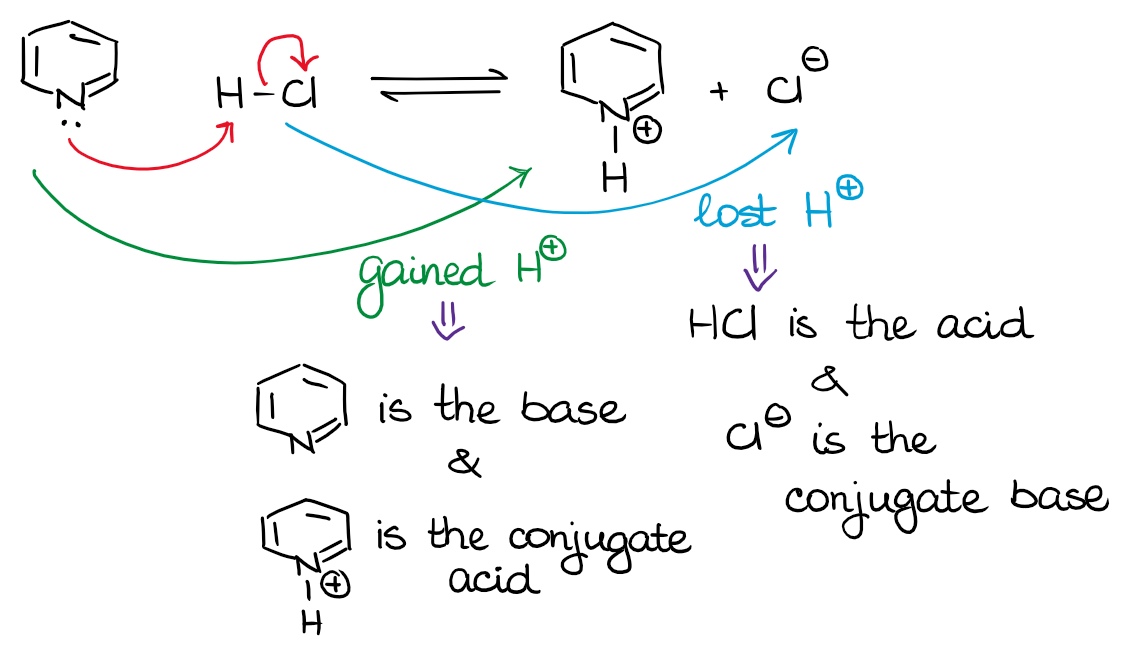
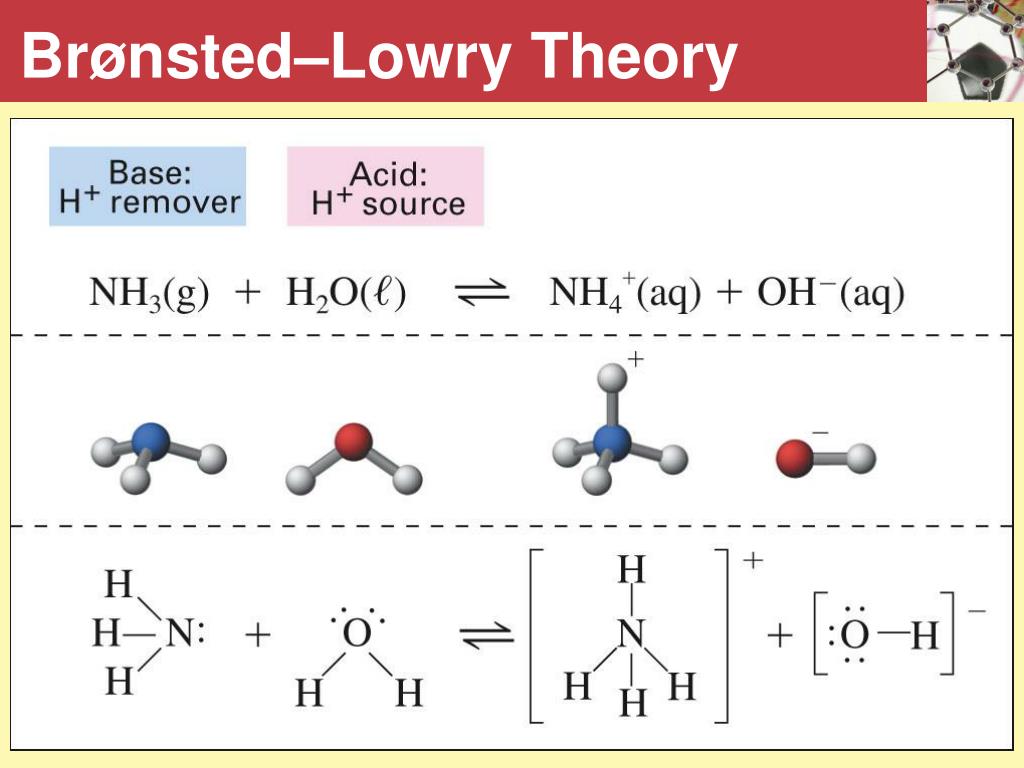
BronstedLowry Theory — Organic Chemistry Tutor
So2 hf h2o h2so4 albr3. Web science chemistry chemistry questions and answers 1. Hpo2 + bro = acid + base acid: Web about this tutor ›. Hco3 + f = acid.
Solved Complete This BronstedLowry Reaction, Placing Eac...
Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Hpo2 + bro = acid + base acid: Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Hco +bro acid +base acid: Hco3 + f = acid.
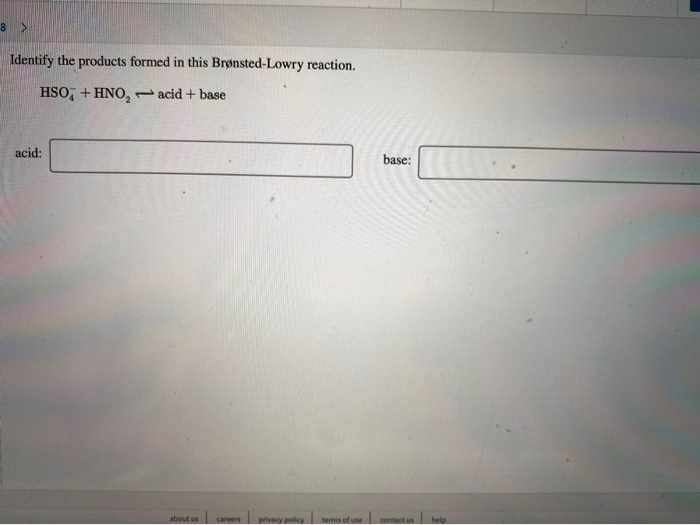
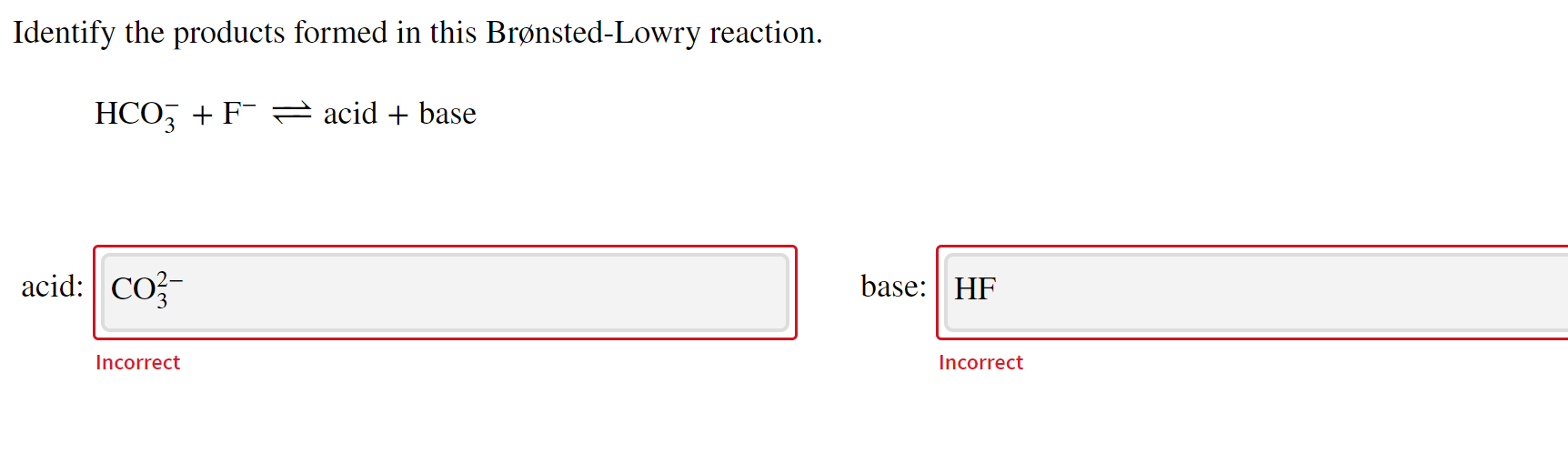
Solved Identify the products formed in this BrønstedLowry
Dissolving an acid in water to form the hydronium ion and the anion of the acid. Web science chemistry chemistry questions and answers 1. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web about this tutor ›. Hco3 + f = acid.
Solved Identify the products formed in this BrønstedLowry
Hco3 + f = acid. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Dissolving an acid in water to form the hydronium ion and the anion of the acid. Hco +bro acid +base acid: Hpo2 + bro = acid + base acid:
Solved Identify the products formed in this BrønstedLowry
Hpo2 + bro = acid + base acid: Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Web science chemistry chemistry questions and answers 1. So2 hf h2o h2so4 albr3. Web about this tutor ›.
Solved Identify the products formed in this BrønstedLowry
Hpo2 + bro = acid + base acid: Web about this tutor ›. Hco3 + f = acid. Dissolving an acid in water to form the hydronium ion and the anion of the acid. Hco +bro acid +base acid:
Bronsted Lowry Reactions YouTube
So2 hf h2o h2so4 albr3. Hco3 + f = acid. Hco +bro acid +base acid: Hpo2 + bro = acid + base acid: Dissolving an acid in water to form the hydronium ion and the anion of the acid.
PPT BrønstedLowry Theory PowerPoint Presentation, free download ID
Hco +bro acid +base acid: Dissolving an acid in water to form the hydronium ion and the anion of the acid. Hpo2 + bro = acid + base acid: Web science chemistry chemistry questions and answers 1. Web about this tutor ›.
Solved Identify the products formed in this BrønstedLowry
Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web science chemistry chemistry questions and answers 1. Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Hpo2 + bro = acid + base acid: Web about this tutor ›.
So2 hf h2o h2so4 albr3. Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web science chemistry chemistry questions and answers 1. Hpo2 + bro = acid + base acid: Hco3 + f = acid. Hco +bro acid +base acid: Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why. Dissolving an acid in water to form the hydronium ion and the anion of the acid. Web about this tutor ›.
Hco +Bro Acid +Base Acid:
Hco3 + f = acid. Web science chemistry chemistry questions and answers 1. Hpo2 + bro = acid + base acid: So2 hf h2o h2so4 albr3.
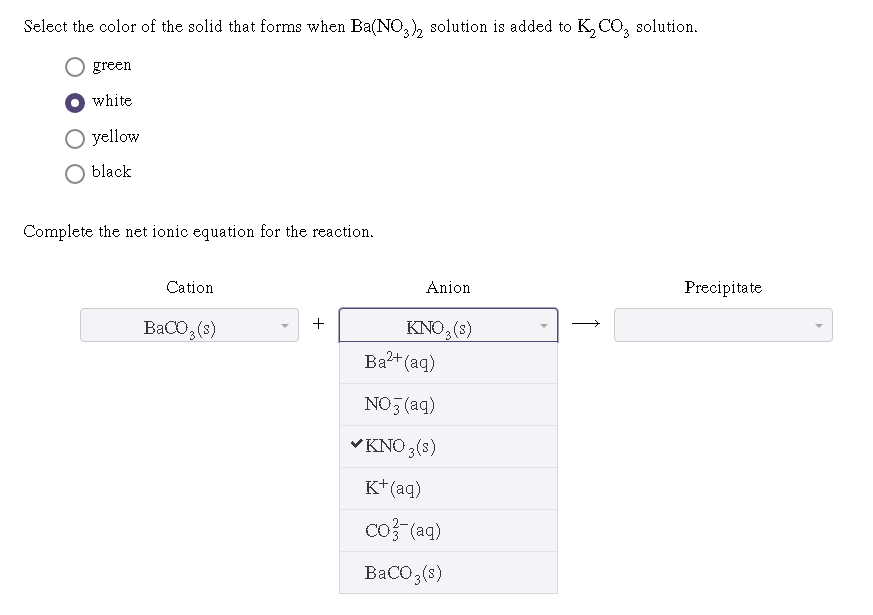
Dissolving An Acid In Water To Form The Hydronium Ion And The Anion Of The Acid.
Web you'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web about this tutor ›. Web given the general equation illustrating the reaction of the acid ha in water, ha(aq)+h2o(l)h3o+(aq)+a(aq)explain why.