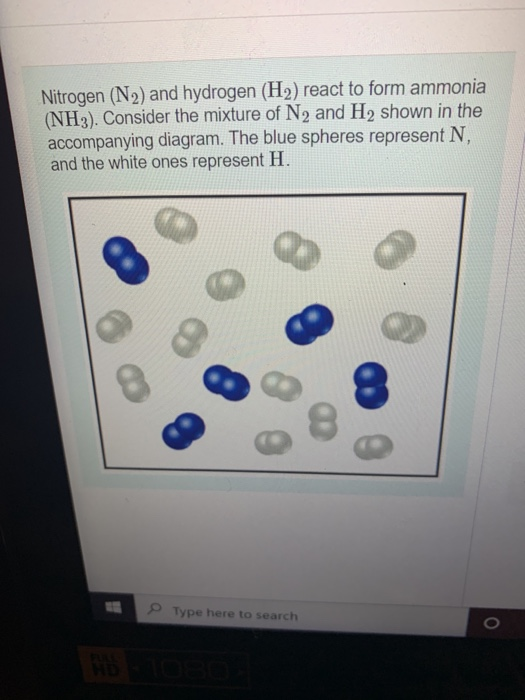
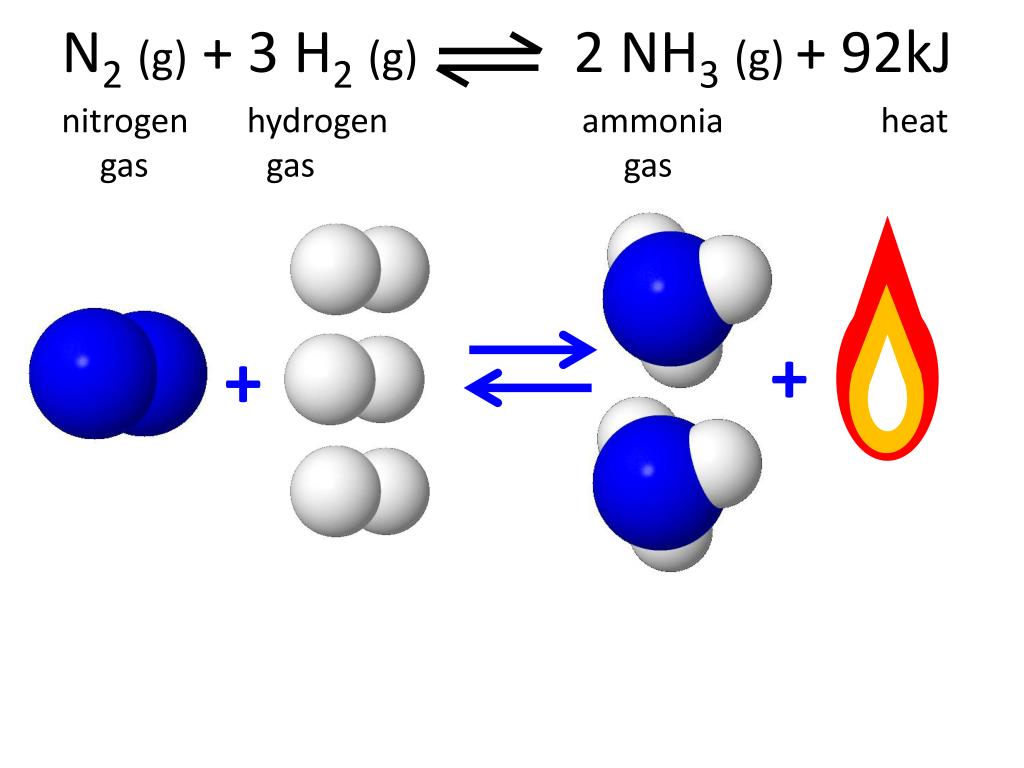
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas. - Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web nitrogen and hydrogen combine to form ammonia via the following reaction: N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web the haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to. I mean to say that, when.
Solved Nitrogen (N2) and hydrogen (H2) react to form ammonia
Web the haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to. Asked • 04/09/19 nitrogen gas and hydrogen gas react to form ammonia according to the following. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. What volume of ammonia would be. Web hydrogen is produced via.
[Solved] Hydrogen gas and nitrogen gas can react to form ammonia
N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. Hydrogen is obtained by reacting natural. Web the haber process is used in the manufacturing of ammonia from nitrogen and hydrogen, and then goes on to. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Asked • 04/09/19 nitrogen gas and hydrogen.
Balanced Chemical Equation For The Synthesis Of Ammonia From Hydrogen
N2 (g) + 3h2 (g) +2nh3 (g) at a. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Asked • 04/09/19 nitrogen gas and hydrogen gas react to form ammonia according to the following. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as;.
Nitrogen gas and hydrogen gas combine to form gaseous ammonia
Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Asked • 04/09/19 nitrogen gas and hydrogen gas react to form ammonia according to the following. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of. What volume of ammonia would be. I mean to say that, when.
Hydrogen Gas and Nitrogen Gas React to Form Ammonia Gas.
The volume of ammonia that would be produced by this reaction if 6.9 m3 of hydrogen were consumed is 4,600.064cm³. Web hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia. N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. Web ammonia is an.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Hydrogen gas and nitrogen gas react to form ammonia gas. Web chemistry questions and answers. I mean to say that, when. N2 (g) + 3h2 (g) +2nh3 (g) at a. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of.
Spice of Lyfe Balanced Chemical Equation For Ammonia Gas
Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Hydrogen gas and nitrogen gas react to form ammonia gas. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride,.
⚗️Question 6 (1 point) Hydrogen gas and nitrogen gas can react to form
1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Web nitrogen and hydrogen combine to form ammonia.
D Question 9 1 pts Nitrogen and hydrogen gas react to form ammonia (NH3
Web the raw materials for the process of making ammonia are hydrogen and nitrogen. Web chemistry questions and answers. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g).
PPT Reaction Rates PowerPoint Presentation, free download ID6516140
N2 (g) + 3h2 (g) +2nh3 (g) at a. N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. Web nitrogen and hydrogen combine to form ammonia via the following reaction: Hydrogen is obtained by reacting natural. The volume of ammonia that would be produced by this reaction if 6.9 m3 of hydrogen were consumed.
Hydrogen gas and nitrogen gas react to form ammonia gas. Asked • 04/09/19 nitrogen gas and hydrogen gas react to form ammonia according to the following. 1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of. Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a. Web the raw materials for the process of making ammonia are hydrogen and nitrogen. Nitrogen and hydrogen gases react to form ammonia gas via the following reaction: N2 (g) + 3h2 (g) +2nh3 (g) at a. Web chemistry questions and answers. Web hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia. N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. The volume of ammonia that would be produced by this reaction if 6.9 m3 of hydrogen were consumed is 4,600.064cm³. Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. Web nitrogen and hydrogen gases react to form ammonia gas as follows: Hydrogen gas and nitrogen gas react to form ammonia gas. Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. I mean to say that, when. Web nitrogen and hydrogen combine to form ammonia via the following reaction: What volume of ammonia would be.
I Mean To Say That, When.
Web ammonia is an inorganic compound of nitrogen and hydrogen with the formula n h 3.a stable binary hydride, and the simplest pnictogen hydride, ammonia is a. Nitrogen gas and hydrogen gas react to form ammonia according to the following thermochemical equation. Web this reaction is the synthesis of ammonia using nitrogen and hydrogen gas. Web hydrogen is produced via steam reforming, followed by an iterative closed cycle to react hydrogen with nitrogen to produce ammonia.
Web Nitrogen And Hydrogen Combine To Form Ammonia Via The Following Reaction:
Web yes, it is absolutely ture, when hydrogen react with nitrogen to form ammonia. Hydrogen is obtained by reacting natural. Hydrogen gas and nitrogen gas react to form ammonia gas. Web the raw materials for the process of making ammonia are hydrogen and nitrogen.
Nitrogen And Hydrogen Gases React To Form Ammonia Gas Via The Following Reaction:
Nitrogen (n2) gas and hydrogen (h2) gas react to form ammonia (nh3) gas. N2 (g) + 3h2 (g) +2nh3 (g) at a. N 2 (g) + 3 h 2 (g) 2 nh 3 (g) at a certain. The volume of ammonia that would be produced by this reaction if 6.9 m3 of hydrogen were consumed is 4,600.064cm³.
Hydrogen Gas And Nitrogen Gas React To Form Ammonia Gas.
1 n 2 (s) + 3 h 2 (g) → 2 nh 3 (g) what mass of. Web the following chemical reaction takes place when hydrogen gas reacts with nitrogen to form ammonia which can be shown as; Web nitrogen and hydrogen gases react to form ammonia gas as follows: Web chemistry questions and answers.