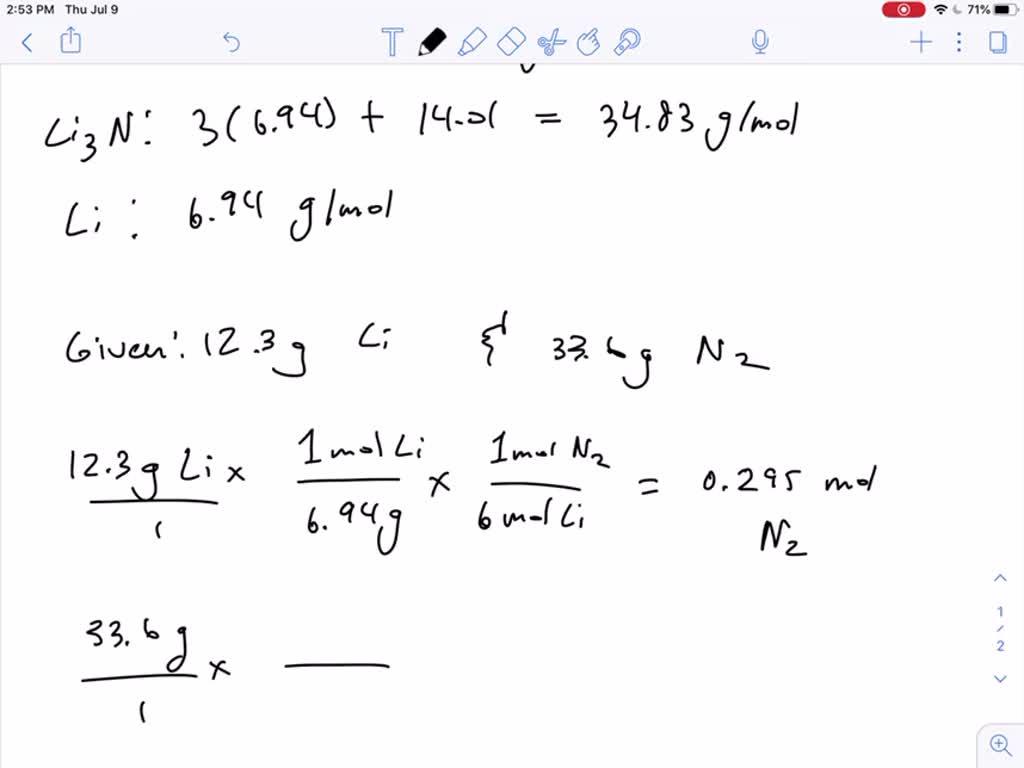How Many Grams Of Li3N Can Be Formed From 1.75 - 6 li (s) + n2 (g) → 2. Web chemistry college answered • expert verified how many grams of lign can be formed from 1.75 moles of li? Assume an excess 'of nitrogen: 6 li(s) + nz(g) 2 lizn(s) 20.3 g. Assume an excess of nitrogen. Web chemistry questions and answers. How many grams of li3n can be. Web we can convert mass of lithium to moles of lithium via the atomic mass of lithium: Web how many grams of li3n can be formed from 1.75 moles of li? How many grams of li3n can be formed from 1.75 moles of li?
Solved 22) How Many Grams Of Li3N Can Be Formed From 1.75...
Web chemistry college answered • expert verified how many grams of lign can be formed from 1.75 moles of li? 1.75 moles li x (2 moles li3n / 6 moles li) =. Web since each molecule of li3n requires 3 atoms of li, that means that from 1.75 moles of li, you can only produce 1.75 / 3. Web chemistry.
When heated lithium reacts with nitrogen to form lith… SolvedLib
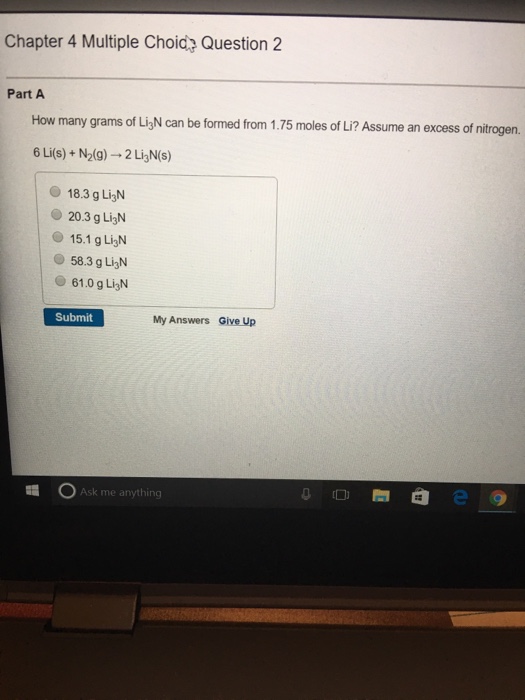
Assume an excess 'of nitrogen: How many grams of li3n can be formed from 1.75 moles of li? Web the balance chemical reaction is: How many grams of li3n can be. 6 li (s) + n2 (g) → 2 li3n (s) we use the amount of the lithium reactant and the.
Solved How many moles of Li3N can be formed by adding 2.00
How many grams of li3n can be formed from 1.75 moles of li? Web we need to calculate the amount of lithium nitride that can be formed. Calculate the number of moles of li3n that can be formed from 1.75 moles of li: 12.1g li (1 mol / 6.941g) = 1.74. Click the card to flip 👆 (1) convert.
Solved How many grams of Li3N can be formed from 1.75 moles
20.3 g first, determine how many moles of li3n you can produce from 1.75. Assume an excess of nitrogen. Assume an excess of nitrogen. How many grams of li3n can be. Web since each molecule of li3n requires 3 atoms of li, that means that from 1.75 moles of li, you can only produce 1.75 / 3.
Solved How Many Grams Of Li_3N Can Be Formed From 1.75 Mo...
6 li (s) + n2 (g) → 2. 1.75 moles li x (2 moles li3n / 6 moles li) =. 6 li (s) + n2 (g) → 2 li3n (s) we use the amount of the lithium reactant and the. Web the balance chemical reaction is: Assume an excess of nitrogen.
How many grams of li3n can be formed from 1.75 moles of li? assume an
How many grams of li3n can be formed from 1.75 moles of li? How many grams of li3n can be. Web how many grams of li3n can be formed from 1.75 moles of li? 6 li(s) + nz(g) 2 lizn(s) 20.3 g. 6 li (s) + n2 (g) → 2 li3n (s) we use the amount of the lithium reactant.
OneClass 1) 07How many grams of Li3N can be formed from 1 75 moles of
How many grams of li3n can be formed from 1.75 moles of li? The balanced chemical equation of the reaction that is in. 1.75 moles li x (2 moles li3n / 6 moles li) =. How many grams of li3n can be formed from 1.75 moles of li? Web since each molecule of li3n requires 3 atoms of li, that.
Solved How many grams of Li_3N can be formed from 1.75 moles
Assume an excess of nitrogen. Web the balance chemical reaction is: 6 li (s) + n2 (g) → 2 li3n (s) we use the amount of the lithium reactant and the. Calculate the number of moles of li3n that can be formed from 1.75 moles of li: Web chemistry questions and answers.
Solved The two beakers below each have added to them the
Click the card to flip 👆 (1) convert. Web since each molecule of li3n requires 3 atoms of li, that means that from 1.75 moles of li, you can only produce 1.75 / 3. Web chemistry questions and answers. How many grams of li3n can be formed from 1.75 moles of li? The balanced chemical equation of the reaction that.
Solved 6) How many grams of Li3N can be formed from 1.75
Assume an excess 'of nitrogen: Web chemistry questions and answers. 20.3 g first, determine how many moles of li3n you can produce from 1.75. Web how many grams of li3n can be formed from 1.75 moles of li? Web we need to calculate the amount of lithium nitride that can be formed.
Assume an excess of nitrogen. Web chemistry questions and answers. 20.3 g first, determine how many moles of li3n you can produce from 1.75. 6 li (s) + n2 (g) → 2 li3n (s) we use the amount of the lithium reactant and the. Web the balance chemical reaction is: Web we need to calculate the amount of lithium nitride that can be formed. 12.1g li (1 mol / 6.941g) = 1.74. How many grams of li3n can be formed from 1.75 moles of li? 6 li (s) + n2 (g) → 2. How many grams of li3n can be. Web we can convert mass of lithium to moles of lithium via the atomic mass of lithium: How many grams of li3n can be formed from 1.75 moles of li? Web how many grams of li3n can be formed from 1.75 moles of li? How many grams of li3n can be formed from 1.75 moles of li? Web 8 people found it helpful. Calculate the number of moles of li3n that can be formed from 1.75 moles of li: How many grams of li3n can be formed from 1.75 moles of li? 6 li(s) + nz(g) 2 lizn(s) 20.3 g. 1.75 moles li x (2 moles li3n / 6 moles li) =. Assume an excess of nitrogen.
Web 2) How Many Grams Of Li3N Can Be Formed From 1.75 Moles Of Li?
Assume an excess 'of nitrogen: Web chemistry college answered • expert verified how many grams of lign can be formed from 1.75 moles of li? Web the balance chemical reaction is: 6 li (s) + n2 (g) → 2.
How Many Grams Of Li3N Can Be Formed From 1.75 Moles Of Li?
Web how many grams of li3n can be formed from 1.75 moles of li? Web 8 people found it helpful. Web we can convert mass of lithium to moles of lithium via the atomic mass of lithium: Assume an excess of nitrogen.
Click The Card To Flip 👆 (1) Convert.
20.3 g first, determine how many moles of li3n you can produce from 1.75. Web since each molecule of li3n requires 3 atoms of li, that means that from 1.75 moles of li, you can only produce 1.75 / 3. How many grams of li3n can be formed from 1.75 moles of li? Web how many grams of li3n can be formed from 1.75 moles of li?
12.1G Li (1 Mol / 6.941G) = 1.74.
Web chemistry questions and answers. 1.75 moles li x (2 moles li3n / 6 moles li) =. Assume an excess of nitrogen. The balanced chemical equation of the reaction that is in.