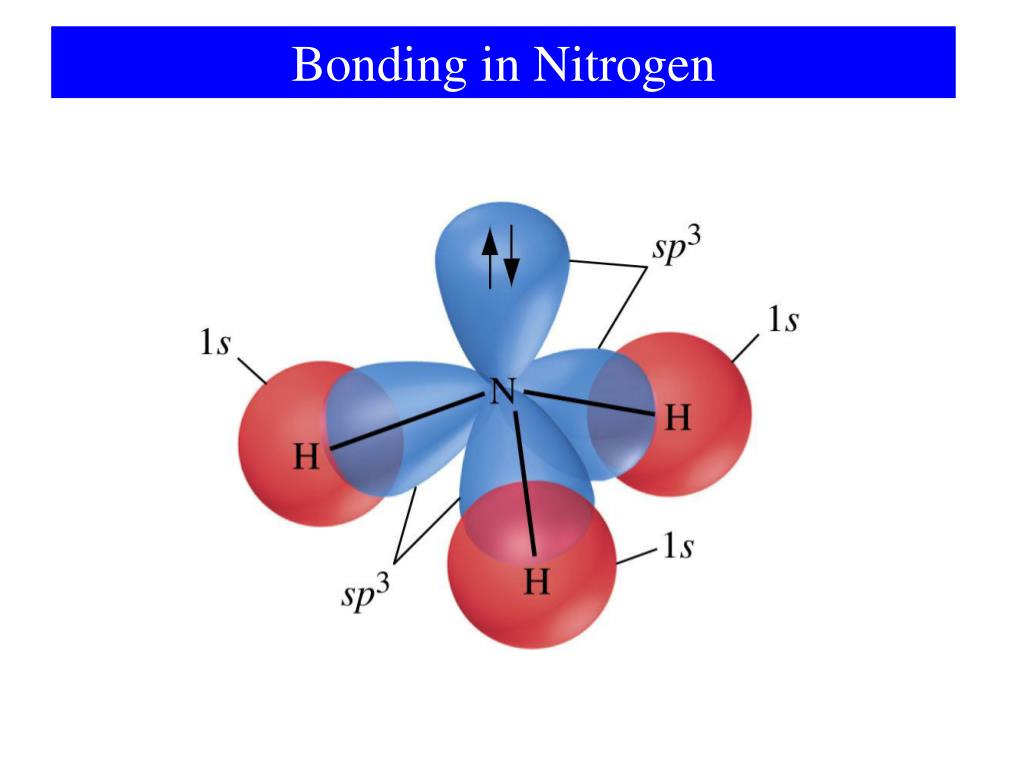
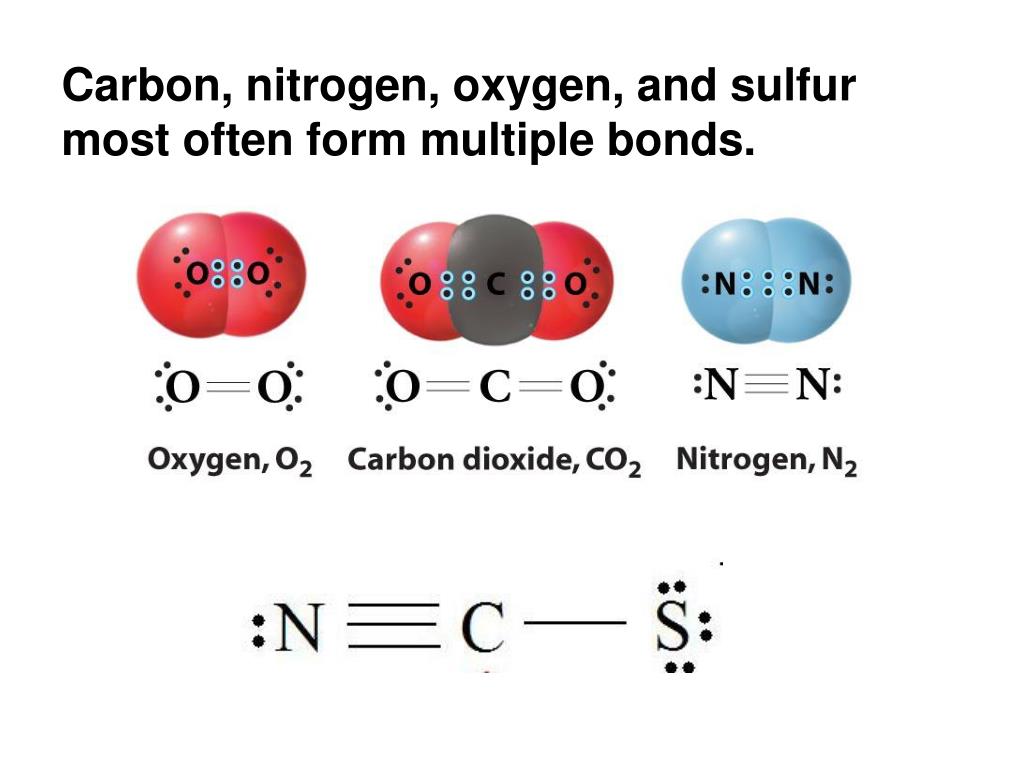
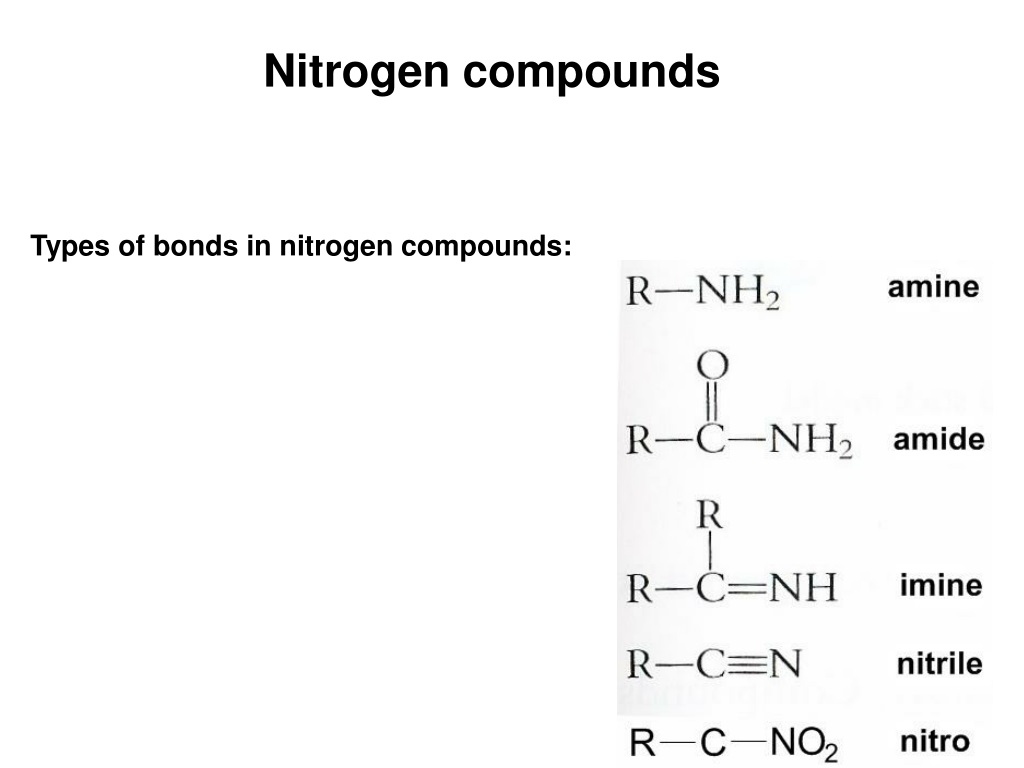
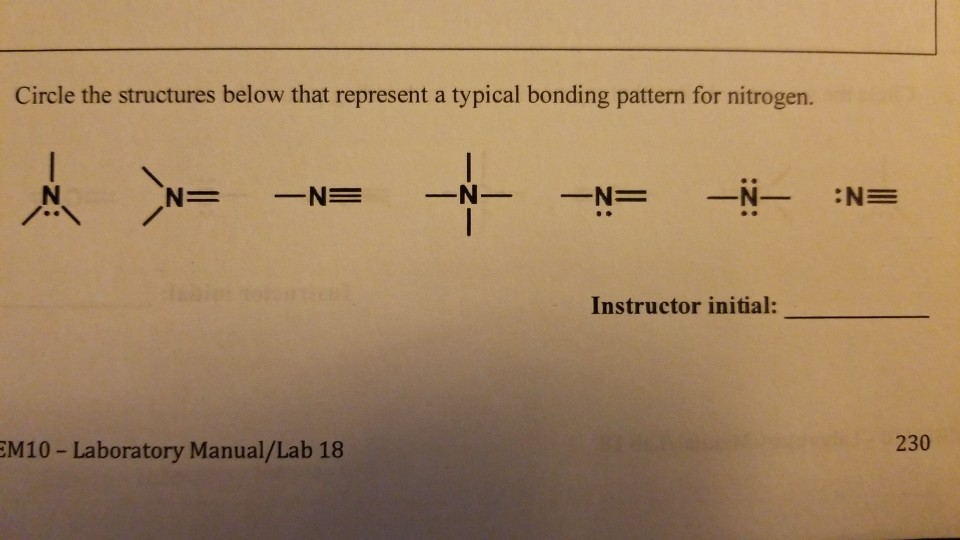
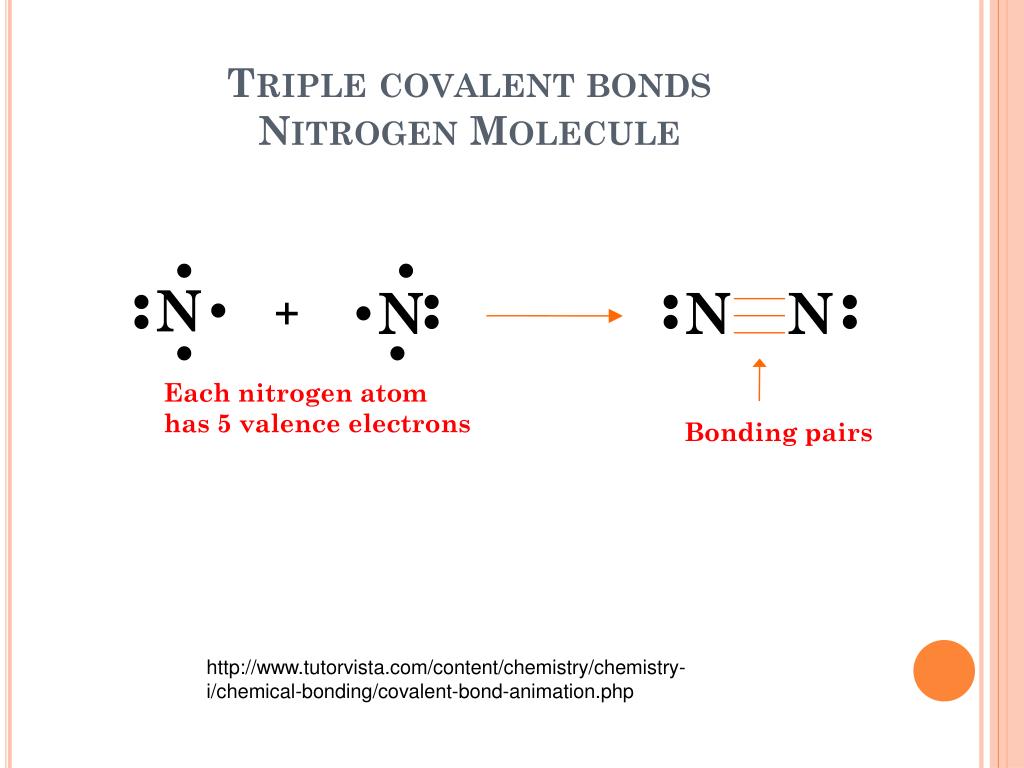
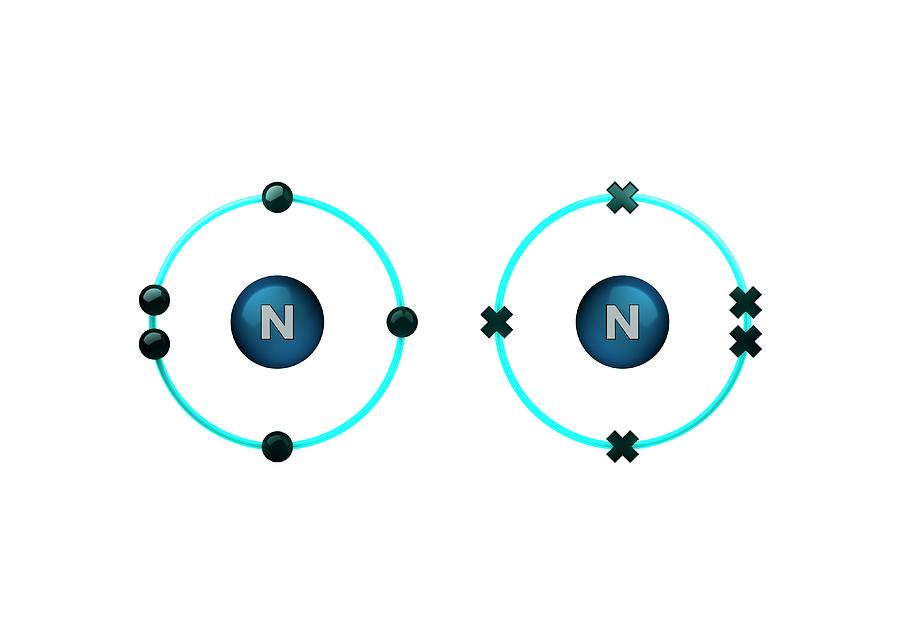
How Many Covalent Bonds Does Nitrogen Form - How many covalent bonds can. Web most often nitrogen forms three covalent bonds. Web two different atoms can also share electrons and form covalent bonds. Nitrogen is in group 5 of the periodic table. This is because it has atomic number 7, so its. Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an. This is because it has atomic number 7, so its electron. Nitrogen has 5 valence electrons. It cannot accept any more. Web theoretically, boron can accommodate five more electrons according to the octet rule, but boron is a very small atom and five.
Case Based MCQ Chemistry in Automobiles For an internal combustion
Nitrogen can form 3 covalent bonds. Web theoretically, boron can accommodate five more electrons according to the octet rule, but boron is a very small atom and five. Sometimes nitrogen will form four. This is because it has atomic number 7, so its electron. Web introduction nitrogen is found to have either 3 or 5 valence electrons and lies at.
PPT General Chemistry PowerPoint Presentation, free download ID4215385
Examples are nh3 (three single bonds) and n2 (one triple bond). Web two different atoms can also share electrons and form covalent bonds. Web therefore, nitrogen can form 3 single covalent bonds. Web how many covalent bonds does nitrogen form in electrically neutral compounds? Nitrogen can form 3 covalent bonds.
PPT Covalent Bonding PowerPoint Presentation, free download ID4132371
Web nitrogen has three electrons in its 2p orbital. Web atoms of different elements. Don’t get confused as in this question single. How many covalent bonds does carbon form in. Polar covalent bonds if the atoms that form a covalent bond are identical, as in h 2, cl 2,.
PPT Organic compounds containing nitrogen and sulfur PowerPoint
Web 2h ( g) h 2 ( g) δ h = −436 kj pure vs. Web atoms of different elements. Web introduction nitrogen is found to have either 3 or 5 valence electrons and lies at the top of group 15 on the periodic. Don’t get confused as in this question single. Polar covalent bonds if the atoms that form.
What Happens When Two Nitrogen Atoms Share Electrons MarisolkruwLee
Nitrogen has 5 valence electrons. It is in the second period of the periodic table and so has its outer electron in the second. There is a quick way to work. This is because it has atomic number 7, so its electron. Web therefore, nitrogen can form 3 single covalent bonds.
How to find Valency? What are valence electrons? Teachoo
Polar covalent bonds if the atoms that form a covalent bond are identical, as in h 2, cl 2,. Web nitrogen has three electrons in its 2p orbital. How many covalent bonds does carbon form in. Web introduction nitrogen is found to have either 3 or 5 valence electrons and lies at the top of group 15 on the periodic..
Solved How many bonds does nitrogen typically form? Does
Web two nitrogen atoms can bond to form a triple covalent bond which will give each individual atom an additional 3 electrons for an. Don’t get confused as in this question single. Web nitrogen typically forms 3 covalent bonds, including in #n_2#. Web atoms of different elements. Web introduction nitrogen is found to have either 3 or 5 valence electrons.
PPT COVALENT BONDING PowerPoint Presentation, free download ID5128236
It is in the second period of the periodic table and so has its outer electron in the second. Web theoretically, boron can accommodate five more electrons according to the octet rule, but boron is a very small atom and five. Two nitrogen atoms will each share three electrons to form. Web two nitrogen atoms can bond to form a.
89. Covalent Bonding(35) MOT(10) Nitrogen molecule. Madoverchemistry
Examples are nh3 (three single bonds) and n2 (one triple bond). Don’t get confused as in this question single. Web nitrogen atoms will form three covalent bonds (also called triple covalent) between two atoms of nitrogen because each nitrogen atom needs three. Sometimes nitrogen will form four. Web two different atoms can also share electrons and form covalent bonds.
Bond Formation In Nitrogen Molecule Photograph by
Don’t get confused as in this question single. Web a nitrogen atom has 5 electrons in its outer shell. There is a quick way to work. Web introduction nitrogen is found to have either 3 or 5 valence electrons and lies at the top of group 15 on the periodic. Web two different atoms can also share electrons and form.
Therefore, it can form three bonds by sharing its three electrons. Don’t get confused as in this question single. If nitrogen is to remain. This is because it has atomic number 7, so its electron. It cannot accept any more. There is a quick way to work. Nitrogen can form 3 covalent bonds. This is because it has atomic number 7, so its. Nitrogen has 5 valence electrons. Web therefore, nitrogen can form 3 single covalent bonds. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Sometimes nitrogen will form four. Web most often nitrogen forms three covalent bonds. Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Examples are nh3 (three single bonds) and n2 (one triple bond). Web a nitrogen atom has 5 electrons in its outer shell. Web nitrogen makes 3 covalent bonds. Web how can you tell the number covalent bonds the atoms of an element can form? Web two different atoms can also share electrons and form covalent bonds. Two nitrogen atoms will each share three electrons to form.
Nitrogen Has 5 Valence Electrons.
Nitrogen can form 3 covalent bonds. Web nitrogen makes 3 covalent bonds. Web 2h ( g) h 2 ( g) δ h = −436 kj pure vs. Examples are nh3 (three single bonds) and n2 (one triple bond).
Don’t Get Confused As In This Question Single.
Therefore, it can form three bonds by sharing its three electrons. Polar covalent bonds if the atoms that form a covalent bond are identical, as in h 2, cl 2,. Web how many covalent bonds does nitrogen form in electrically neutral compounds? Sometimes nitrogen will form four.
If Nitrogen Is To Remain.
Web group 5a (15) elements such as nitrogen have five valence electrons in the atomic lewis symbol: Two nitrogen atoms will each share three electrons to form. Web therefore, nitrogen can form 3 single covalent bonds. This is because it has atomic number 7, so its.
It Cannot Accept Any More.
How many covalent bonds can. Web two different atoms can also share electrons and form covalent bonds. Web atoms of different elements. Will form either one, two, three or four covalent bonds with other atoms.