How Many Bonds Does Bromine Form - However, it can also form multiple bonds with other. Which of the following situations meet the bonding requirement for carbon atoms. Web how many bonds can bromine make? There is a single bond between. Web bromine will normally form one covalent bond. Web the first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands,. Web bromine does not generally form double bonds, but there are rare cases in which it does. 6 which elements tend to form covalent bonds? Two single bonds and a double bond. Molybdenum bromine oxygen potassium nitrogen how.
Collins New GCSE Science Gateway B page 112
Web bromine is capable of forming one bond when it is in its elemental form. Web the first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands,. Group 5a form 3 bonds; Web when bromine atoms form covalent bonds with other atoms, they share their electrons equally. Web bromine does.
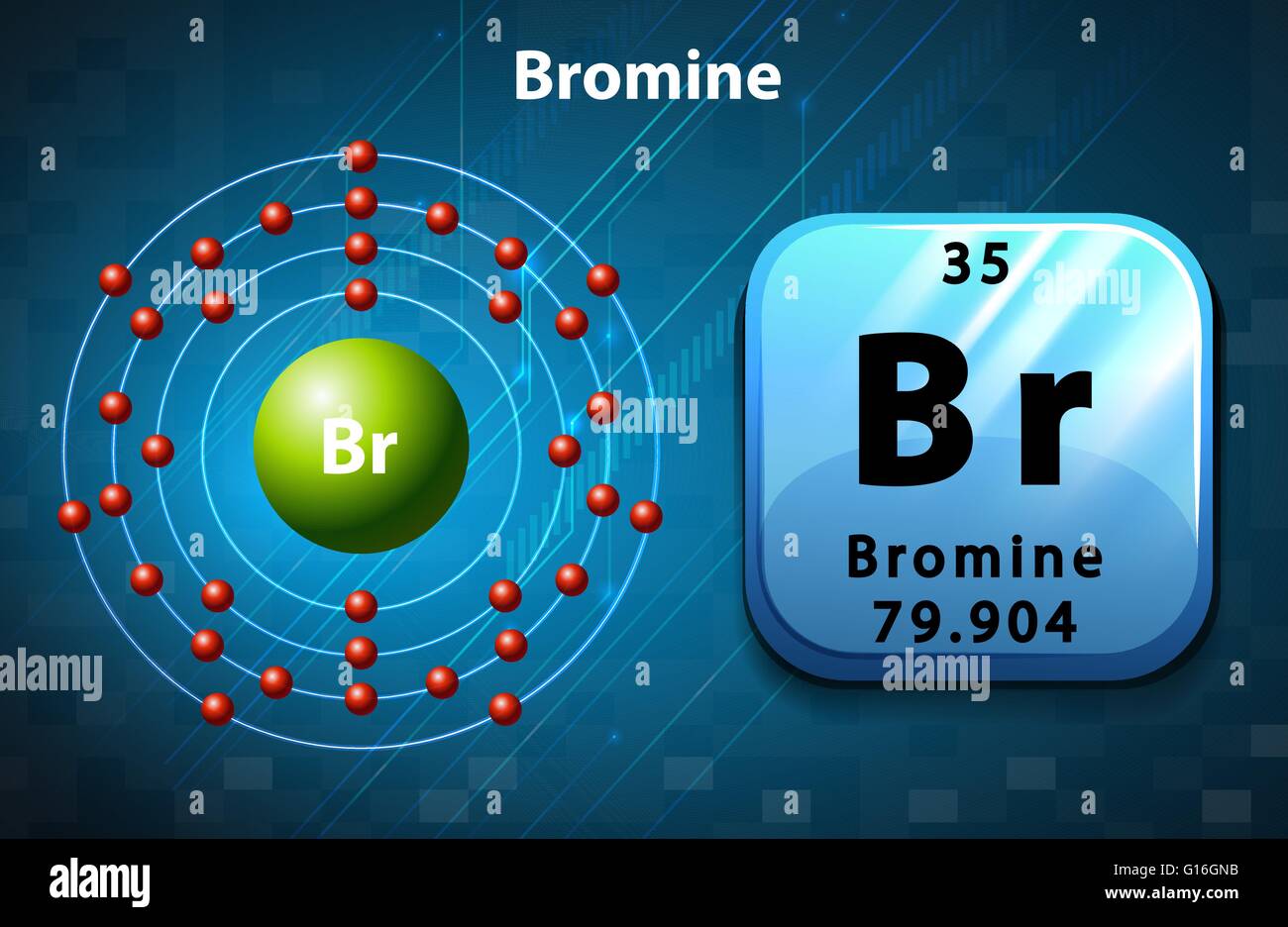
Bromine Periodic Table and Atomic Properties
Create your account view this answer the. Web bromine is a diatomic molecule and contains only bromine atoms. There is a single bond between. 6 which elements tend to form covalent bonds? Molybdenum bromine oxygen potassium nitrogen how.
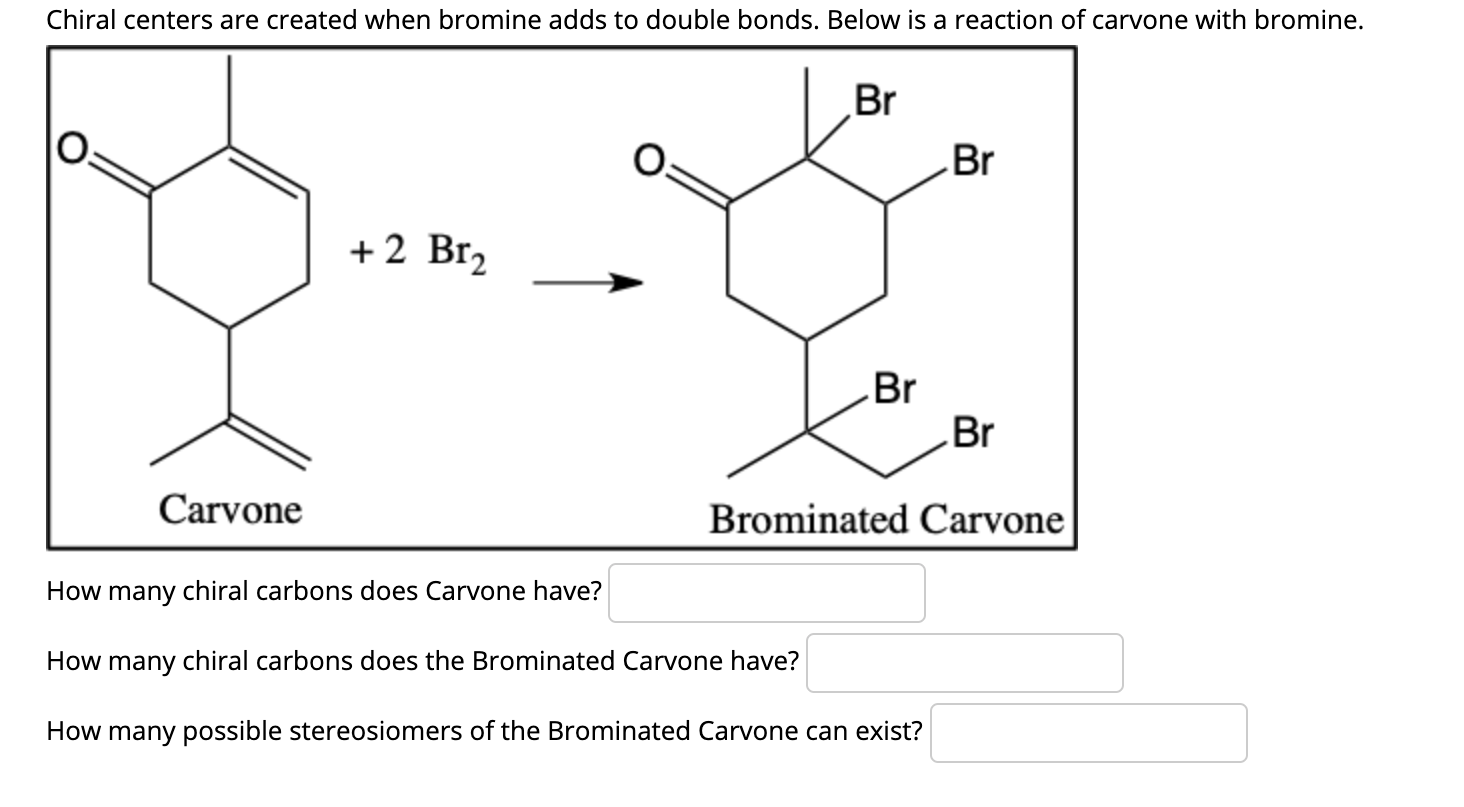
Solved Chiral centers are created when bromine adds to
Which of the following situations meet the bonding requirement for carbon atoms. Web how many bonds can bromine make? Web diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Sources, facts, uses, scarcity (sri), podcasts,. Molybdenum bromine oxygen potassium nitrogen how.
Bromine Formula Symbol of Bromine, Density, Charge Embibe
Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web bromine does not generally form double bonds, but there are rare cases in which it does. Web typically, the atoms of group 4a form 4 covalent bonds; Sources, facts, uses, scarcity (sri), podcasts,. Web when bromine atoms form covalent bonds with other atoms, they share.
Bromine Electron Configuration (Br) with Orbital Diagram
Web which two elements are components of many organic molecules? Web the atomic number of bromine is 35, and the atomic weight is 79.904. Two single bonds and a double bond. Web bromine is a diatomic molecule and contains only bromine atoms. Group 6a form 2 bonds;
Symbol and electron diagram for Bromine illustration Stock Vector Image
Web diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Web how many bonds can bromine make? 6 which elements tend to form covalent bonds? Two single bonds and a double bond.
PPT Chemistry Unit 6 PowerPoint Presentation, free download ID6652385
Create your account view this answer the. Web how many bonds can bromine make? 6 which elements tend to form covalent bonds? Web bromine is a diatomic molecule and contains only bromine atoms. Web bromine will normally form one covalent bond.
Atom Diagrams Electron Configurations of the Elements
Group 5a form 3 bonds; Create your account view this answer the. Which of the following situations meet the bonding requirement for carbon atoms. Web bromine is a diatomic molecule and contains only bromine atoms. Web bromine will normally form one covalent bond.
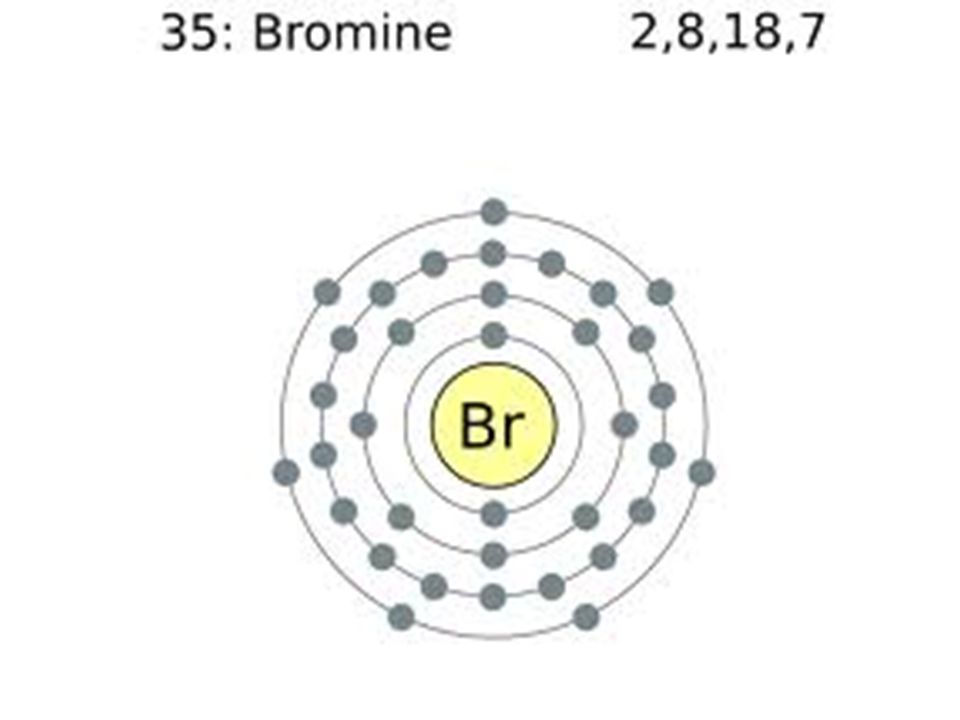
How Can We Find A Electron Configuration For Bromine (Br)
Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all. Web which two elements are components of many organic molecules? There is a single bond between. Web the atomic number of bromine is 35, and the atomic weight is 79.904. Which of the following situations meet the.
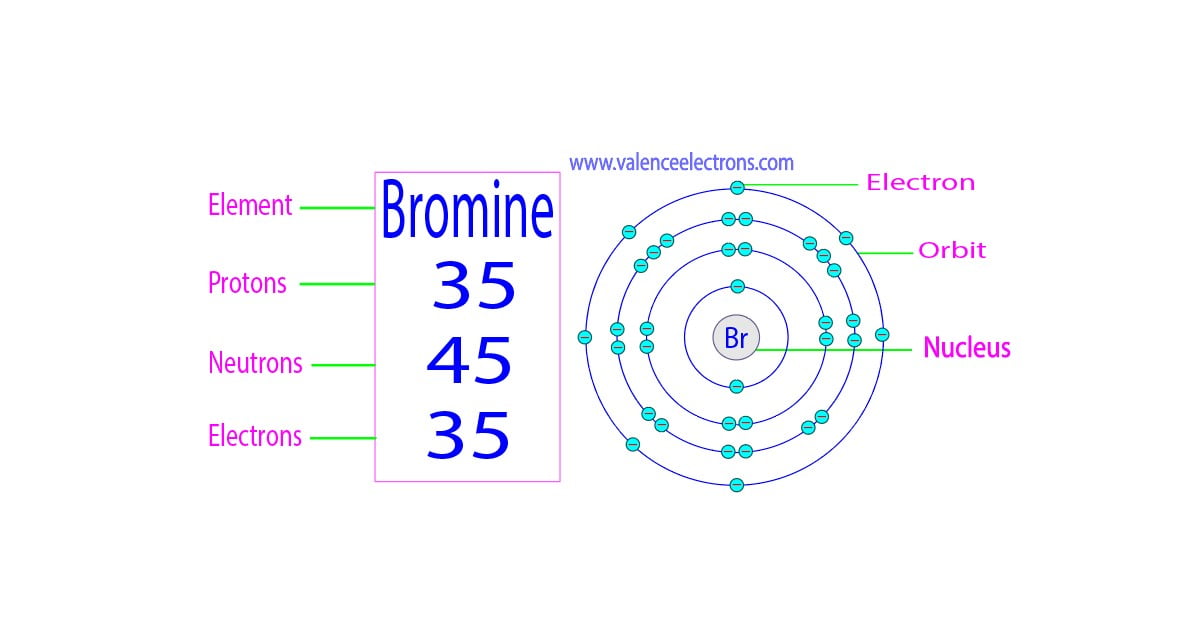
How many protons, neutrons and electrons does bromine have?
Web through sharing, the iodine atom now has access to eight valence electrons, as does the bromine atom. Web how many bonds can bromine make? Web which two elements are components of many organic molecules? However, it can also form multiple bonds with other. Web bromine is a diatomic molecule and contains only bromine atoms.
Sources, facts, uses, scarcity (sri), podcasts,. Web the atomic number of al is 13, and its electronic configuration is 1s22p22p63s23p1. Web the first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands,. Create your account view this answer the. Two single bonds and a double bond. Web bromine is a diatomic molecule and contains only bromine atoms. Web through sharing, the iodine atom now has access to eight valence electrons, as does the bromine atom. Web bromine will normally form one covalent bond. Web when bromine atoms form covalent bonds with other atoms, they share their electrons equally. Web if either iodine or bromine were to given up valence electrons to form a cation, they would have to give up all. There is a single bond between. Bromine, which belongs to group 17 and period four of the periodic table, has. Bromine, which belongs to group 17 and period four of the periodic. Web bromine is capable of forming one bond when it is in its elemental form. Web typically, the atoms of group 4a form 4 covalent bonds; Molybdenum bromine oxygen potassium nitrogen how. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. Web which two elements are components of many organic molecules? Which of the following situations meet the bonding requirement for carbon atoms. Web bromine will normally form one covalent bond.
Web Bromine Will Normally Form One Covalent Bond.
Which of the following situations meet the bonding requirement for carbon atoms. Web through sharing, the iodine atom now has access to eight valence electrons, as does the bromine atom. 6 which elements tend to form covalent bonds? Web how many bonds can bromine make?
However, It Can Also Form Multiple Bonds With Other.
Web typically, the atoms of group 4a form 4 covalent bonds; Molybdenum bromine oxygen potassium nitrogen how. Bromine, which belongs to group 17 and period four of the periodic table, has. Web when bromine atoms form covalent bonds with other atoms, they share their electrons equally.
Web Bromine Will Normally Form One Covalent Bond.
Web diatomic bromine is a chemical compound when two neutral bromine atoms combine by a covalent bond. Web which two elements are components of many organic molecules? Two single bonds and a double bond. Group 6a form 2 bonds;
Create Your Account View This Answer The.
Web the first ionization energy of bromine is high, and compounds containing bromine in positive oxidation numbers are stabilized by appropriate ligands,. Web the atomic number of bromine is 35, and the atomic weight is 79.904. Web let's illustrate how a covalent bond forms between iodine and bromine, with the understanding that each atom only. There is a single bond between.




:max_bytes(150000):strip_icc()/Bromine-58b601f93df78cdcd83d2817.jpg)